正在加载图片...
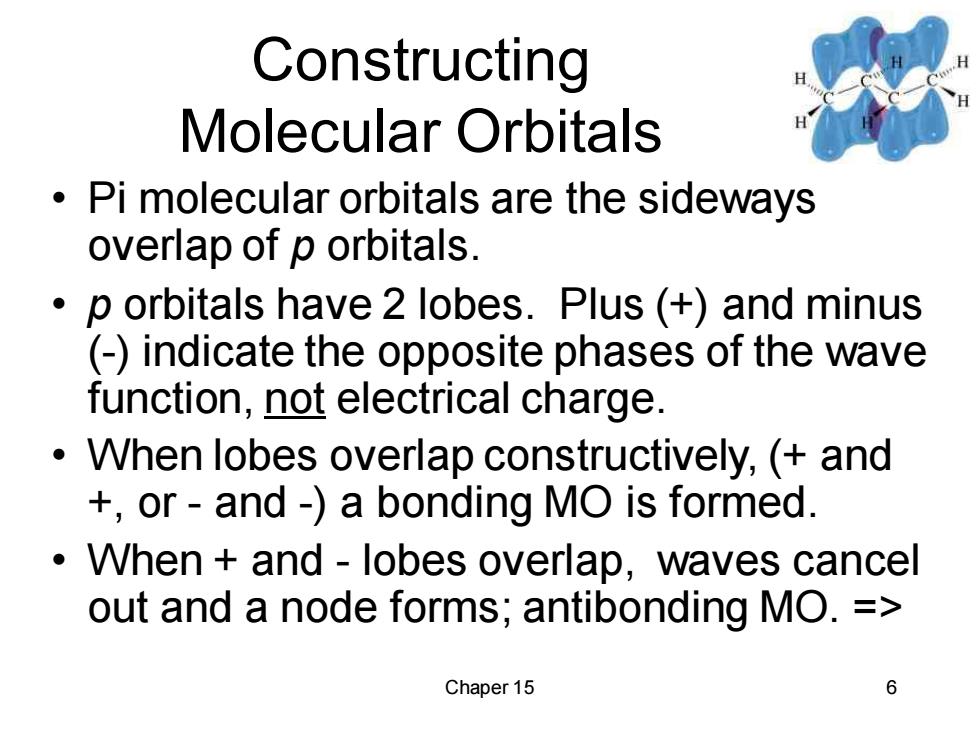
Constructing Molecular Orbitals Pi molecular orbitals are the sideways overlap of p orbitals. p orbitals have 2 lobes.Plus (+)and minus (-)indicate the opposite phases of the wave function,not electrical charge. When lobes overlap constructively,(and +or-and-)a bonding MO is formed. When and-lobes overlap,waves cancel out and a node forms;antibonding MO.= Chaper 15 6Chaper 15 6 Constructing Molecular Orbitals • Pi molecular orbitals are the sideways overlap of p orbitals. • p orbitals have 2 lobes. Plus (+) and minus (-) indicate the opposite phases of the wave function, not electrical charge. • When lobes overlap constructively, (+ and +, or - and -) a bonding MO is formed. • When + and - lobes overlap, waves cancel out and a node forms; antibonding MO. =>