正在加载图片...
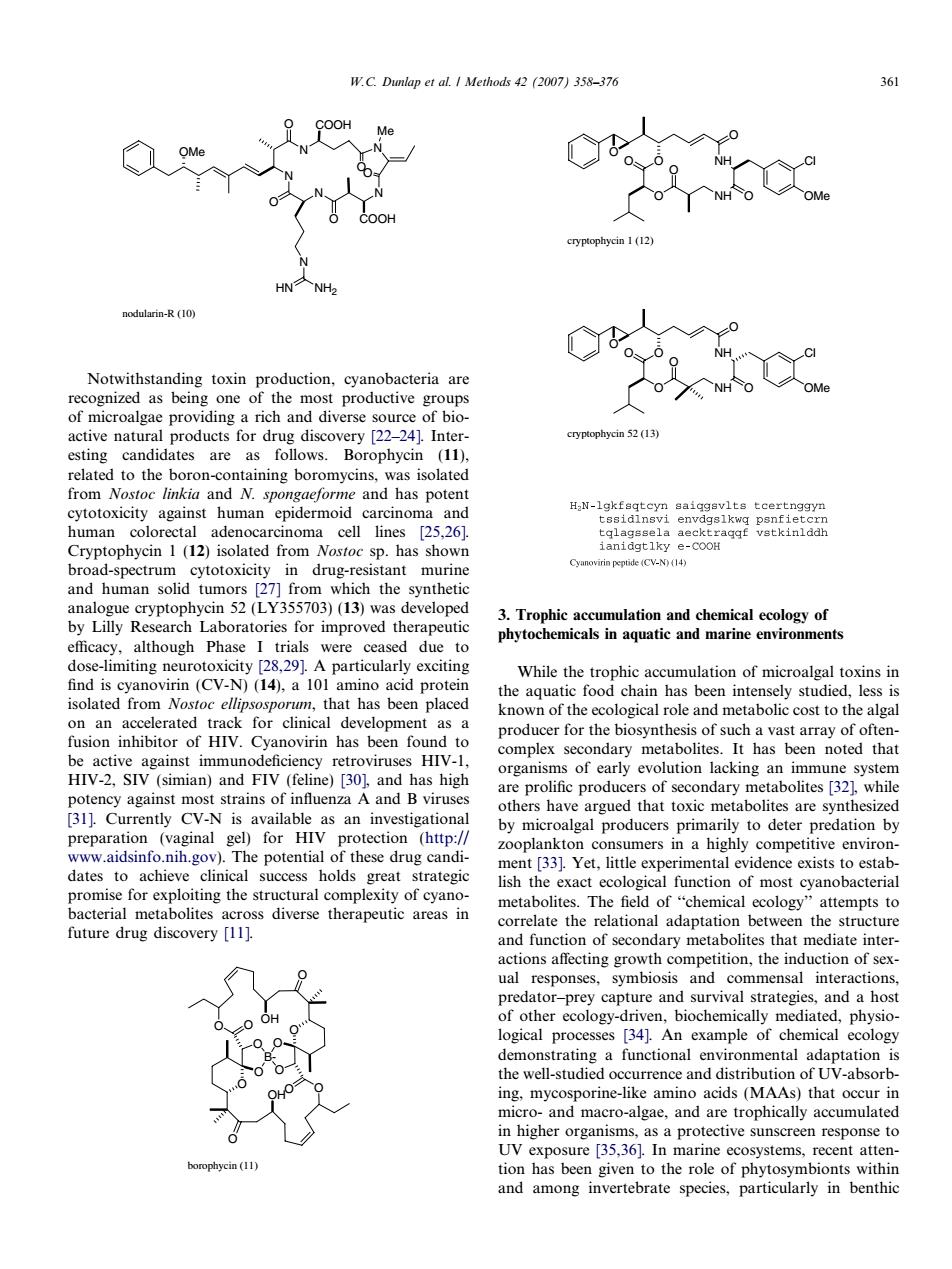
W.C.Dulap et al.I Methods 42(2007)358-37 6 COOH NH> odularin-R (10) Notwithstanding toxin production,cyanobacteria are an erse 2-24m cryptophycin 52(13) esting candidates are as follows.Borophycin (11). om Nosto and N.spon and has poten H N-lgkfaqtcyn saiqgevlts tcertnggyn Cryptophycin 1(12)isolated from Nostoc sp.has showr broadspetniaomoricit7tnodnfi eCV-N)(14) stant murin umo52355703)13 h phytoch efficacy,although Phase due to limiting ne 14 While the trophic accumulation of microalgal toxins in that has been placed the aquatic food chain has been intensely studied,less is as a nown of the ecological role and metabolic cost to the algal Cyanovirin has been producer for the ast array of organisms of early evolution lacking an immune system potency against most strains of influenza A and B viruses are prolific producers of secondary metabolites [321 while thers hav hat toxic m [31].Currently CV-N is available oltes are synthe as an investigationa ooplankton consumers in a high dates to achieve clinical success holds great strategic xact ecol or exploiting the structural complexity diverse therapeutic areas metab the and function of secondary metabolites that mediate inter- tition,the induction of sex logical processes [34].An example of chemical ecology a functional as a protective sunscreen response to marine ecosystems and among invertebrate species.particularly in benthic N N N N N HN NH2 Me N O COOH OO OMe O COOH O nodularin-R (10) Notwithstanding toxin production, cyanobacteria are recognized as being one of the most productive groups of microalgae providing a rich and diverse source of bioactive natural products for drug discovery [22–24]. Interesting candidates are as follows. Borophycin (11), related to the boron-containing boromycins, was isolated from Nostoc linkia and N. spongaeforme and has potent cytotoxicity against human epidermoid carcinoma and human colorectal adenocarcinoma cell lines [25,26]. Cryptophycin 1 (12) isolated from Nostoc sp. has shown broad-spectrum cytotoxicity in drug-resistant murine and human solid tumors [27] from which the synthetic analogue cryptophycin 52 (LY355703) (13) was developed by Lilly Research Laboratories for improved therapeutic efficacy, although Phase I trials were ceased due to dose-limiting neurotoxicity [28,29]. A particularly exciting find is cyanovirin (CV-N) (14), a 101 amino acid protein isolated from Nostoc ellipsosporum, that has been placed on an accelerated track for clinical development as a fusion inhibitor of HIV. Cyanovirin has been found to be active against immunodeficiency retroviruses HIV-1, HIV-2, SIV (simian) and FIV (feline) [30], and has high potency against most strains of influenza A and B viruses [31]. Currently CV-N is available as an investigational preparation (vaginal gel) for HIV protection (http:// www.aidsinfo.nih.gov). The potential of these drug candidates to achieve clinical success holds great strategic promise for exploiting the structural complexity of cyanobacterial metabolites across diverse therapeutic areas in future drug discovery [11]. O O O B- O O O O O O OH O O O OH borophycin (11) O O O NH NH O Cl OMe O O O cryptophycin 1 (12) O O NH NH O Cl OMe O O O O cryptophycin 52 (13) 3. Trophic accumulation and chemical ecology of phytochemicals in aquatic and marine environments While the trophic accumulation of microalgal toxins in the aquatic food chain has been intensely studied, less is known of the ecological role and metabolic cost to the algal producer for the biosynthesis of such a vast array of oftencomplex secondary metabolites. It has been noted that organisms of early evolution lacking an immune system are prolific producers of secondary metabolites [32], while others have argued that toxic metabolites are synthesized by microalgal producers primarily to deter predation by zooplankton consumers in a highly competitive environment [33]. Yet, little experimental evidence exists to establish the exact ecological function of most cyanobacterial metabolites. The field of ‘‘chemical ecology’’ attempts to correlate the relational adaptation between the structure and function of secondary metabolites that mediate interactions affecting growth competition, the induction of sexual responses, symbiosis and commensal interactions, predator–prey capture and survival strategies, and a host of other ecology-driven, biochemically mediated, physiological processes [34]. An example of chemical ecology demonstrating a functional environmental adaptation is the well-studied occurrence and distribution of UV-absorbing, mycosporine-like amino acids (MAAs) that occur in micro- and macro-algae, and are trophically accumulated in higher organisms, as a protective sunscreen response to UV exposure [35,36]. In marine ecosystems, recent attention has been given to the role of phytosymbionts within and among invertebrate species, particularly in benthic W.C. Dunlap et al. / Methods 42 (2007) 358–376 361