正在加载图片...
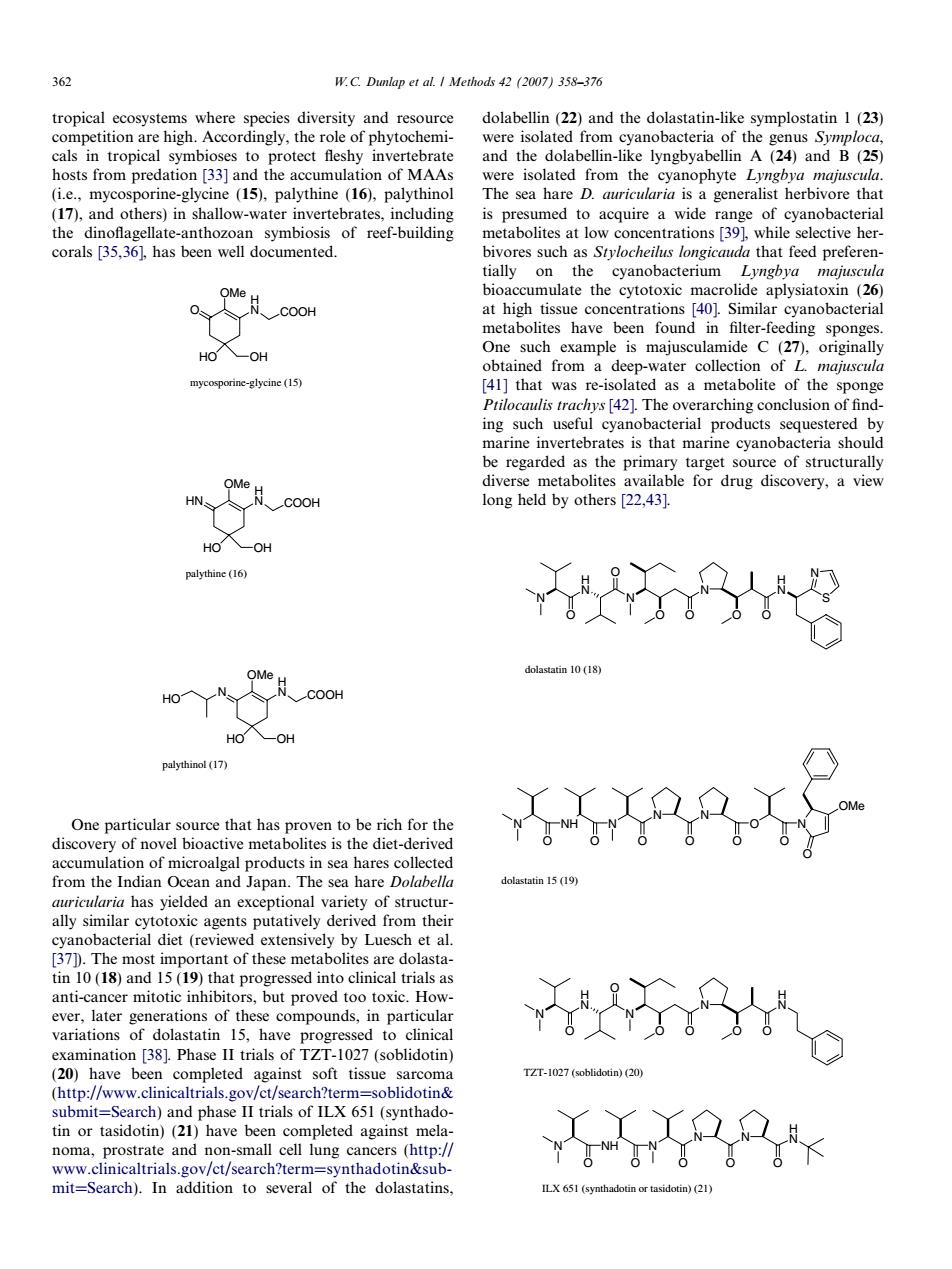
362 W.C.Dumlap et al.I Methods 42(2007)358-376 tropical ecosystems where species diversity and resource dolabellin (22)and the dolastatin-like symplostatin 1(23) 331 to protect eonTCba ad the B lyngbya (ie.mycosporine-gycine (15).palythine (16).palythinol The sea hare D.herbivore that (17),and others)in shallow-water invertebrates,including is presumed to acquire a wide range of cyanobacterial tially on the bioaccumulate the cytotoxic macrolide aplysiatoxin (26) One maiuscula obtained from a deep-water collection ofL majuscula ine-glycine(1 [41]that was re-isolated as a metabolite of the sponge [421 The overarching conclusio One that has proven to be rich for the accumulation of microalgal products in sea hares collected from the Indian Ocean and Japan.The sea hare Dolabella al similar Ytotoiso their c agents putatvely 37).The most important of these metabolites are dolasta- tin 10(18)and 15(19)that progressed into clinical tria ls as anti-cancer mitotic in but proved variations or dolastatin 15.have pro ressed to clinica examination [38].Phase II trials of TZT-1027(soblidotin) 。 ZT-2)(2 tin or tasidotin)(21)have been completed against mela and non-smal cell lung cancers (http:/tropical ecosystems where species diversity and resource competition are high. Accordingly, the role of phytochemicals in tropical symbioses to protect fleshy invertebrate hosts from predation [33] and the accumulation of MAAs (i.e., mycosporine-glycine (15), palythine (16), palythinol (17), and others) in shallow-water invertebrates, including the dinoflagellate-anthozoan symbiosis of reef-building corals [35,36], has been well documented. O H N HO OMe OH COOH mycosporine-glycine (15) H N HO OMe OH HN COOH palythine (16) H N HO OMe OH N COOH HO palythinol (17) One particular source that has proven to be rich for the discovery of novel bioactive metabolites is the diet-derived accumulation of microalgal products in sea hares collected from the Indian Ocean and Japan. The sea hare Dolabella auricularia has yielded an exceptional variety of structurally similar cytotoxic agents putatively derived from their cyanobacterial diet (reviewed extensively by Luesch et al. [37]). The most important of these metabolites are dolastatin 10 (18) and 15 (19) that progressed into clinical trials as anti-cancer mitotic inhibitors, but proved too toxic. However, later generations of these compounds, in particular variations of dolastatin 15, have progressed to clinical examination [38]. Phase II trials of TZT-1027 (soblidotin) (20) have been completed against soft tissue sarcoma (http://www.clinicaltrials.gov/ct/search?term=soblidotin& submit=Search) and phase II trials of ILX 651 (synthadotin or tasidotin) (21) have been completed against melanoma, prostrate and non-small cell lung cancers (http:// www.clinicaltrials.gov/ct/search?term=synthadotin&submit=Search). In addition to several of the dolastatins, dolabellin (22) and the dolastatin-like symplostatin 1 (23) were isolated from cyanobacteria of the genus Symploca, and the dolabellin-like lyngbyabellin A (24) and B (25) were isolated from the cyanophyte Lyngbya majuscula. The sea hare D. auricularia is a generalist herbivore that is presumed to acquire a wide range of cyanobacterial metabolites at low concentrations [39], while selective herbivores such as Stylocheilus longicauda that feed preferentially on the cyanobacterium Lyngbya majuscula bioaccumulate the cytotoxic macrolide aplysiatoxin (26) at high tissue concentrations [40]. Similar cyanobacterial metabolites have been found in filter-feeding sponges. One such example is majusculamide C (27), originally obtained from a deep-water collection of L. majuscula [41] that was re-isolated as a metabolite of the sponge Ptilocaulis trachys [42]. The overarching conclusion of finding such useful cyanobacterial products sequestered by marine invertebrates is that marine cyanobacteria should be regarded as the primary target source of structurally diverse metabolites available for drug discovery, a view long held by others [22,43]. N H N O N O O N O O O H N S N dolastatin 10 (18) N NH N O O N O O N O O N O OMe O dolastatin 15 (19) N H N O N O O N O O O H N TZT-1027 (soblidotin) (20) N NH N O O N O O N O H N ILX 651 (synthadotin or tasidotin) (21) 362 W.C. Dunlap et al. / Methods 42 (2007) 358–376