正在加载图片...
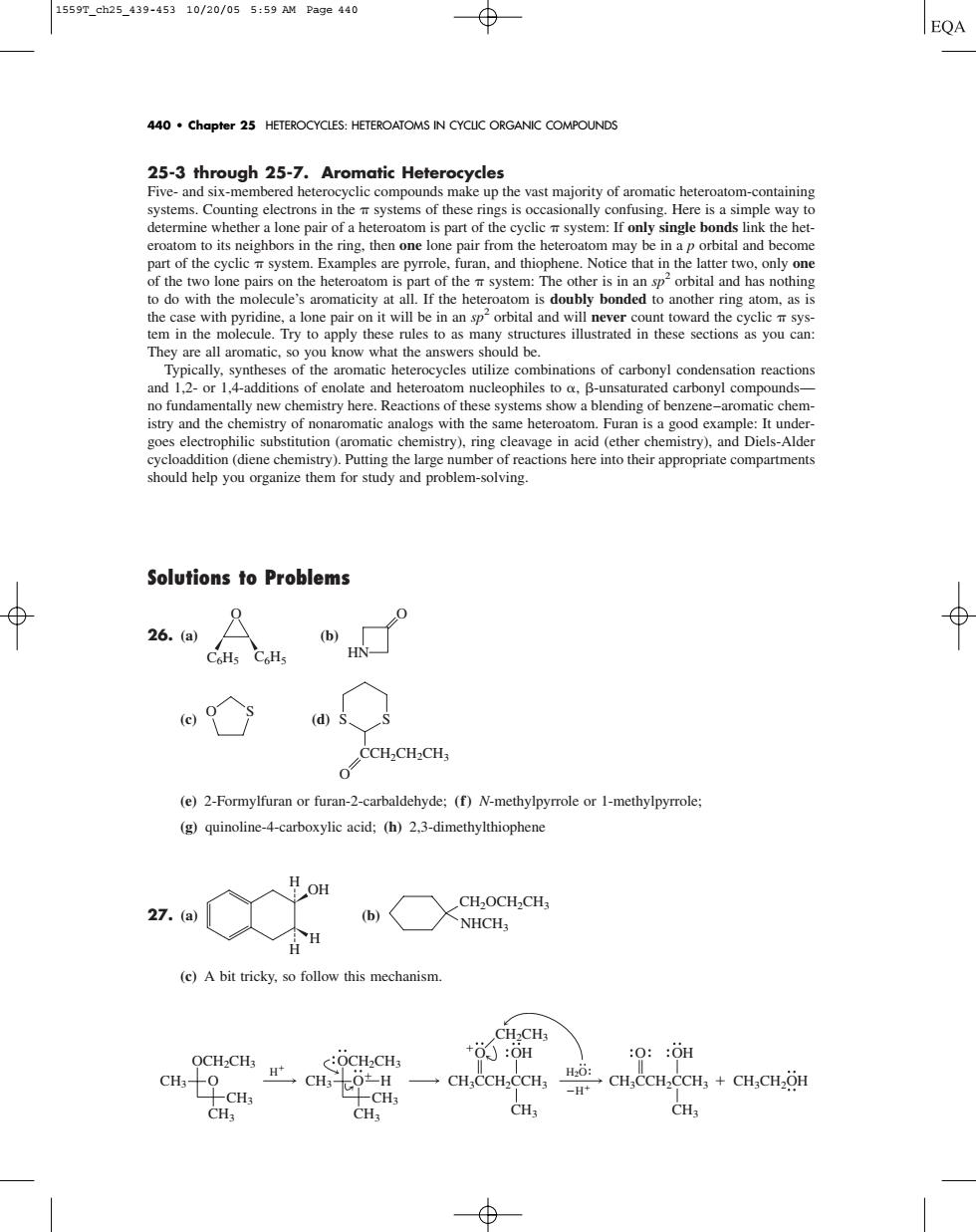
1559Tch25_439-45310/20/055:59 M Page4a0 EQA 440.chapter 25 HETEROCYCLES:HETEROATOMS IN CYCLC ORGANIC COMPOUNDS 25-3 through 25-7.Aromatic Hete rocycles tainin determine whethera lone pair of a heteroatom is part of the cyclicsystem:If only single bonds link the het e l pair from he n of the two lone pairson the heter atom is art of thesystem:The other is in an sporbital and has nothin to do with the lecule's aromaticity at a I the hete y bonded to another ring ato om,as i 2 structures illustrated in these scctions as you can They are aromatic,so you know what the ans wers should be inations of carbonyl c and 2-d no fundamentally new chemistry here.Reactions of these systems show a blending of benzene-aromatic chem istry and the logs with the same heteroatom.Furan is a good exa &cioddioadeeChemsLHmguheiagembeodeaconshecrehoihcrpopaieconpateas should help you organize them for study and problem-solving. Solutions to Problems 0 26.(a) (b CoHs CoHs HN (ds、s (e)2-Formylfuran or furan-2-carbaldehyde:(f)N-methylpyrrole or 1-methylpyrrole: (g)quinoline-4-carboxylic acid:(h)2.3-dimethylthiophene 27.(a b,〈XNHC, (e)A bit tricky.so follow this mechanism OCH-CHs +6:0n :0::0H cH % →CH.CCH,CCH3 CH.CCH.CCH+CH.CH2OH CH25-3 through 25-7. Aromatic Heterocycles Five- and six-membered heterocyclic compounds make up the vast majority of aromatic heteroatom-containing systems. Counting electrons in the systems of these rings is occasionally confusing. Here is a simple way to determine whether a lone pair of a heteroatom is part of the cyclic system: If only single bonds link the heteroatom to its neighbors in the ring, then one lone pair from the heteroatom may be in a p orbital and become part of the cyclic system. Examples are pyrrole, furan, and thiophene. Notice that in the latter two, only one of the two lone pairs on the heteroatom is part of the system: The other is in an sp2 orbital and has nothing to do with the molecule’s aromaticity at all. If the heteroatom is doubly bonded to another ring atom, as is the case with pyridine, a lone pair on it will be in an sp2 orbital and will never count toward the cyclic system in the molecule. Try to apply these rules to as many structures illustrated in these sections as you can: They are all aromatic, so you know what the answers should be. Typically, syntheses of the aromatic heterocycles utilize combinations of carbonyl condensation reactions and 1,2- or 1,4-additions of enolate and heteroatom nucleophiles to , -unsaturated carbonyl compounds— no fundamentally new chemistry here. Reactions of these systems show a blending of benzene–aromatic chemistry and the chemistry of nonaromatic analogs with the same heteroatom. Furan is a good example: It undergoes electrophilic substitution (aromatic chemistry), ring cleavage in acid (ether chemistry), and Diels-Alder cycloaddition (diene chemistry). Putting the large number of reactions here into their appropriate compartments should help you organize them for study and problem-solving. Solutions to Problems 26. (a) (b) (c) (d) (e) 2-Formylfuran or furan-2-carbaldehyde; (f) N-methylpyrrole or 1-methylpyrrole; (g) quinoline-4-carboxylic acid; (h) 2,3-dimethylthiophene 27. (a) (b) (c) A bit tricky, so follow this mechanism. H H2O OCH2CH3 CH3 CH3 CH3 O OCH2CH3 CH3 CH3 CH3 O H CH3CCH2CCH3 CH3 CH2CH3 O OH CH3CCH2CCH3 CH3CH2OH CH3 O OH H CH2OCH2CH3 NHCH3 H H H OH CCH2CH2CH3 O S S O S HN O C6H5 C6H5 O 440 • Chapter 25 HETEROCYCLES: HETEROATOMS IN CYCLIC ORGANIC COMPOUNDS 1559T_ch25_439-453 10/20/05 5:59 AM Page 440��