正在加载图片...
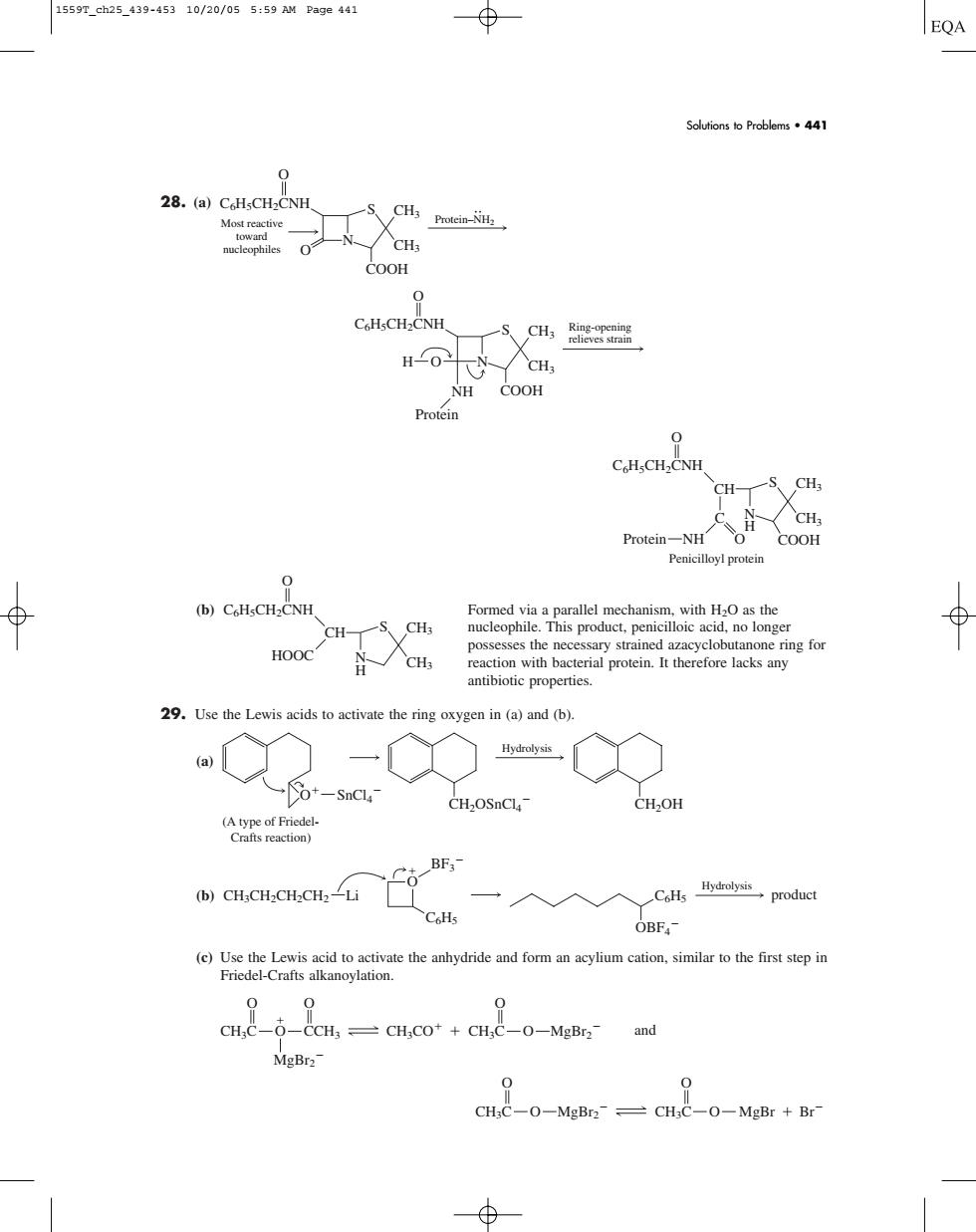
1559T_ch25_439-45310/20/055:59 AM Page441 ⊕ EQA Soutionso Problems441 28.(a)Ch.CH-CNH COOH C HsCH-CNH HO CH NH COOH C.H.CH- CH-S CH Protein-NHH 0 (b)CoHsCH2CNH Formed viaa parallel mechanism,with Has the HOOC ing for antibiotic properties. 29.Use the Lewis acids to activate the ring oxygen in (a)and (b). o+-sncla (e)Use the Lewis acid to activate the anhydride and form an acylium cation.similar to the first step in Friedel-Crafts alkanoylation. 0 0 CHC-0-CCH,CHCo*+CH;c-0-MgBr2-and MgBr2- CH:C-0-MgBr2-=CHC-0-MgBr Br 28. (a) (b) Formed via a parallel mechanism, with H2O as the nucleophile. This product, penicilloic acid, no longer possesses the necessary strained azacyclobutanone ring for reaction with bacterial protein. It therefore lacks any antibiotic properties. 29. Use the Lewis acids to activate the ring oxygen in (a) and (b). (a) (b) (c) Use the Lewis acid to activate the anhydride and form an acylium cation, similar to the first step in Friedel-Crafts alkanoylation. and MgBr2 O CH3C MgBr Br CH3C O O O MgBr2 MgBr2 CH3CO O CH3C O CCH3 CH3C O O O C6H5 BF3 Hydrolysis C6H5 OBF4 product O CH3CH2CH2CH2 Li Hydrolysis SnCl4 CH2OSnCl4 CH2OH O (A type of FriedelCrafts reaction) CH3 CH3 S N H HOOC CH C6H5CH2CNH O COOH CH3 CH3 S C N H Protein NH O Penicilloyl protein CH C6H5CH2CNH O Ring-opening relieves strain COOH CH3 CH3 S H O N NH Protein C6H5CH2CNH O Protein–NH2 COOH CH3 CH3 S N O Most reactive toward nucleophiles C6H5CH2CNH O Solutions to Problems • 441 1559T_ch25_439-453 10/20/05 5:59 AM Page 441