正在加载图片...
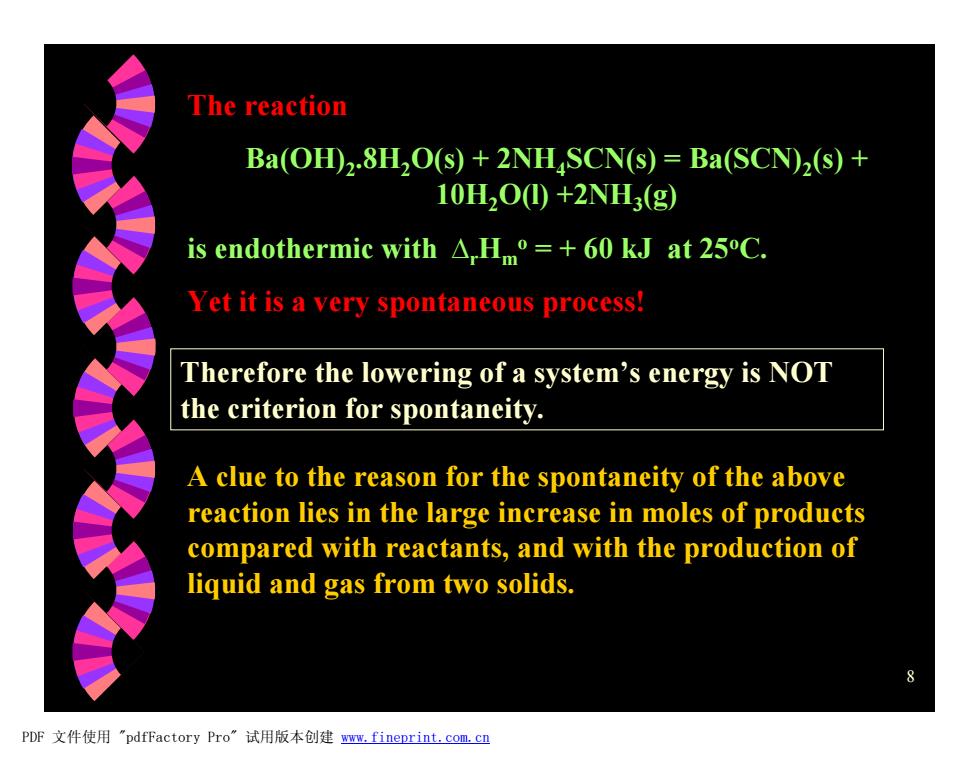
The reaction Ba(OH)2.8H,O(s)+2NHSCN(s)=Ba(SCN)2(s)+ 10H2O0+2NH3(g) is endothermic with A,Hm=+60 kJ at 25C. Yet it is a very spontaneous process! Therefore the lowering of a system's energy is NOT the criterion for spontaneity. A clue to the reason for the spontaneity of the above reaction lies in the large increase in moles of products compared with reactants,and with the production of liquid and gas from two solids. PDF文件使用"pdfFactory Pro”试用版本创建ww.fineprint.com,cn8 The reaction Ba(OH)2 .8H2O(s) + 2NH4SCN(s) = Ba(SCN)2 (s) + 10H2O(l) +2NH3 (g) is endothermic with DrHm o = + 60 kJ at 25oC. Yet it is a very spontaneous process! Therefore the lowering of a system’s energy is NOT the criterion for spontaneity. A clue to the reason for the spontaneity of the above reaction lies in the large increase in moles of products compared with reactants, and with the production of liquid and gas from two solids. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn