The Second Law of Thermodynamics Part I PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,cn
1 The Second Law of Thermodynamics Part I PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

Contents The direction of spontaneous change .Concentrating on the system .Combining the First and Second Laws .Properties of the Gibbs energy 2 PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
2 Contents • The direction of spontaneous change •Concentrating on the system •Combining the First and Second Laws •Properties of the Gibbs energy PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn ÿ
The first law of thermodynamics states that when one form of energy is converted to another,the total energy is conserved.It does not indicate any other restriction on this process.However,we know that many processes have a natural direction,and it is with the question of direction that the second law is concerned. The purpose of this chapter is to explain in the origin of the spontaneity of physical and chemical change. PDF文件使用"pdfFactory Pro”试用版本创建www.fineprint.com.cn
3 The first law of thermodynamics states that when one form of energy is converted to another, the total energy is conserved. It does not indicate any other restriction on this process. However, we know that many processes have a natural direction, and it is with the question of direction that the second law is concerned. The purpose of this chapter is to explain in the origin of the spontaneity of physical and chemical change. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

Spontaneous processes Introduction occur without any outside intervention. Some processes in Nature are SPONTANEOUS. Other processes do not occur spontaneously and we have to force them to happen. e.g.,Place a hot body next to a cold body.The hot body transfers heat to the cold.We never observe the hot body getting hotter and the cold body getting colder! A book on a table can spontaneously fall to the floor. We never observe a book on the floor spontaneously arising! However,neither of these highly improbable events is forbidden by the First Law of Thermodynamics! PDF文件使用"pdfFactory Pro”试用版本创建iw,fineprint.com,c四
4 Introduction Some processes in Nature are SPONTANEOUS. Other processes do not occur spontaneously and we have to force them to happen. e.g., Place a hot body next to a cold body. The hot body transfers heat to the cold. We never observe the hot body getting hotter and the cold body getting colder! A book on a table can spontaneously fall to the floor. We never observe a book on the floor spontaneously arising! However, neither of these highly improbable events is forbidden by the First Law of Thermodynamics! Spontaneous processes occur without any outside intervention. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn Ì
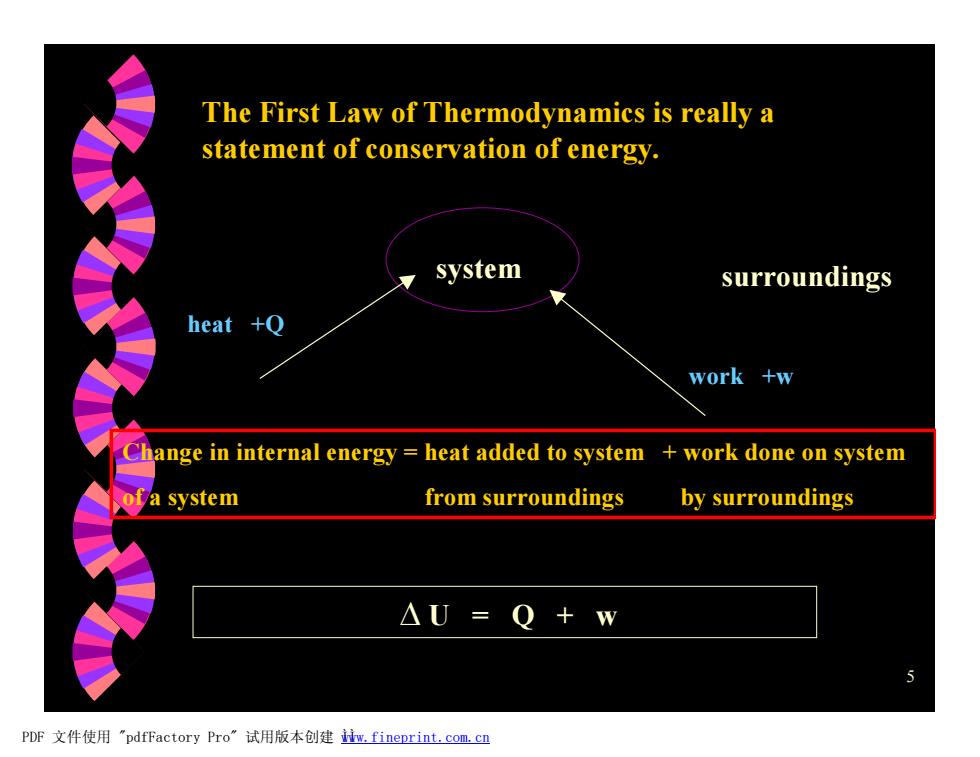
The First Law of Thermodynamics is really a statement of conservation of energy. system surroundings heat +Q work +w Change in internal energy heat added to system work done on system of a system from surroundings by surroundings △U=Q+w PDF文件使用"pdfFactory Pro”试用版本创建iw,fineprint.com.cn
5 The First Law of Thermodynamics is really a statement of conservation of energy. system surroundings heat +Q work +w Change in internal energy = heat added to system + work done on system of a system from surroundings by surroundings D U = Q + w PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn Ì
The quantities Q and w transferred to a system depend on how the change was brought about. However,the change in internal energy,A U,depends ONLY on the initial and final states and NOT on how the change was brought about. The same change in U can be brought about through heat only (w=0)or work only (Q=0)or any arbitrary combination of Q and w whose sum equals A U. In chemistry,the most common change is one in which the pressure is kept constant (generally atmospheric pressure).Here, Qp heat added to system under constant pressure =A H (enthalpy change). 6 PDF文件使用"pdfFactory Pro”试用版本创建w,fineprint.com,c四
6 The quantities Q and w transferred to a system depend on how the change was brought about. However, the change in internal energy, D U , depends ONLY on the initial and final states and NOT on how the change was brought about. The same change in U can be brought about through heat only (w=0) or work only (Q=0) or any arbitrary combination of Q and w whose sum equals D U . In chemistry, the most common change is one in which the pressure is kept constant (generally atmospheric pressure). Here, Qp heat added to system under constant pressure = D H (enthalpy change). PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn
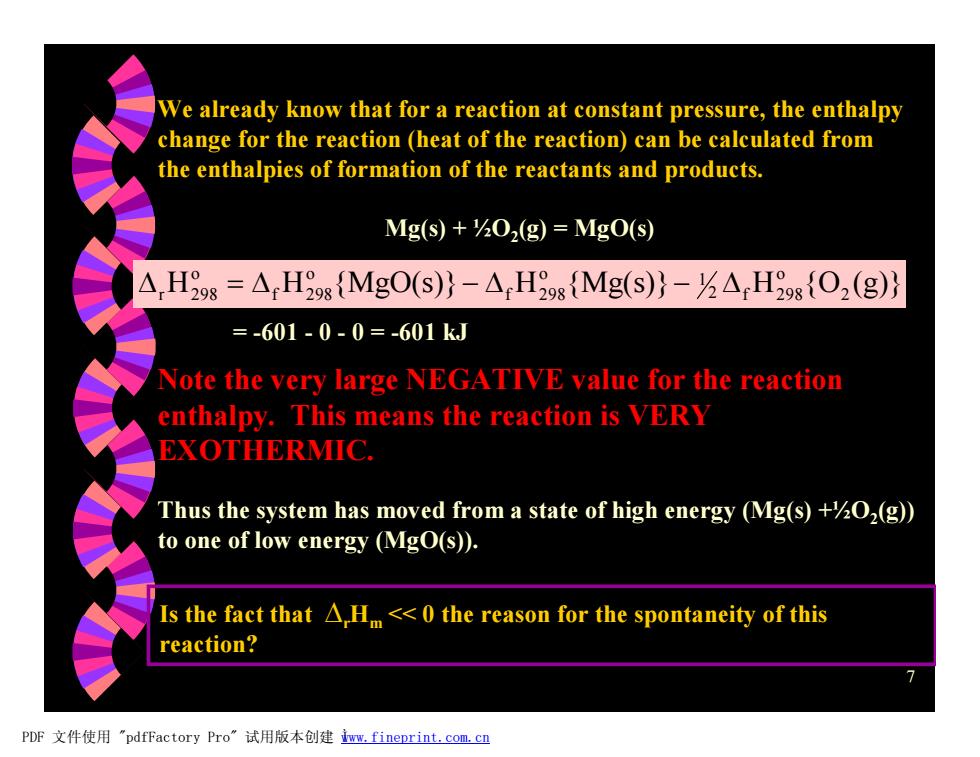
We already know that for a reaction at constant pressure,the enthalpy change for the reaction (heat of the reaction)can be calculated from the enthalpies of formation of the reactants and products. Mg(s)+0,(g)=MgO(s) △,Hs=△H9g{MgO(S)}-△,Hg{Mg(S)}-△Hg{O2(g)} =-601-0-0=-601kJ Note the very large NEGATIVE value for the reaction enthalpy.This means the reaction is VERY EXOTHERMIC. Thus the system has moved from a state of high energy (Mg(s)+202(g)) to one of low energy (MgO(s)). Is the fact that A,Hm<<0 the reason for the spontaneity of this reaction? PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint..com,c四
7 We already know that for a reaction at constant pressure, the enthalpy change for the reaction (heat of the reaction) can be calculated from the enthalpies of formation of the reactants and products. Mg(s) + ½O2 (g) = MgO(s) H H {MgO(s)} H {Mg(s)} H {O (g)} 2 o 2 f 298 1 o f 298 o f 298 o Dr 298 = D - D - D = -601 - 0 - 0 = -601 kJ Note the very large NEGATIVE value for the reaction enthalpy. This means the reaction is VERY EXOTHERMIC. Thus the system has moved from a state of high energy (Mg(s) +½O2 (g)) to one of low energy (MgO(s)). Is the fact that DrHm << 0 the reason for the spontaneity of this reaction? PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn
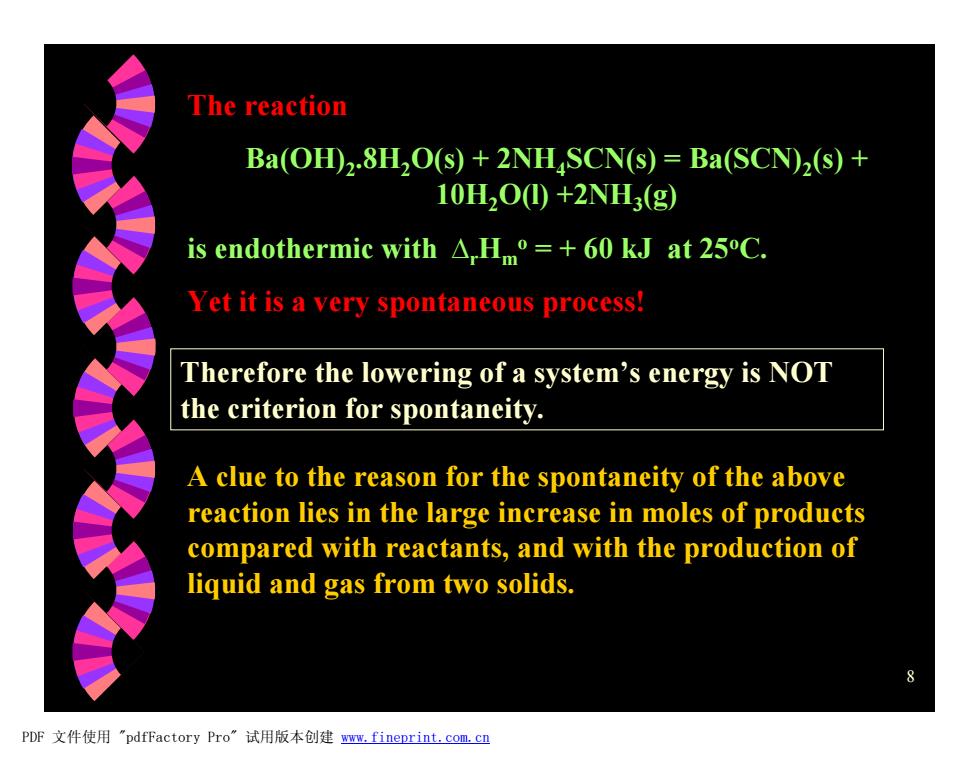
The reaction Ba(OH)2.8H,O(s)+2NHSCN(s)=Ba(SCN)2(s)+ 10H2O0+2NH3(g) is endothermic with A,Hm=+60 kJ at 25C. Yet it is a very spontaneous process! Therefore the lowering of a system's energy is NOT the criterion for spontaneity. A clue to the reason for the spontaneity of the above reaction lies in the large increase in moles of products compared with reactants,and with the production of liquid and gas from two solids. PDF文件使用"pdfFactory Pro”试用版本创建ww.fineprint.com,cn
8 The reaction Ba(OH)2 .8H2O(s) + 2NH4SCN(s) = Ba(SCN)2 (s) + 10H2O(l) +2NH3 (g) is endothermic with DrHm o = + 60 kJ at 25oC. Yet it is a very spontaneous process! Therefore the lowering of a system’s energy is NOT the criterion for spontaneity. A clue to the reason for the spontaneity of the above reaction lies in the large increase in moles of products compared with reactants, and with the production of liquid and gas from two solids. PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn
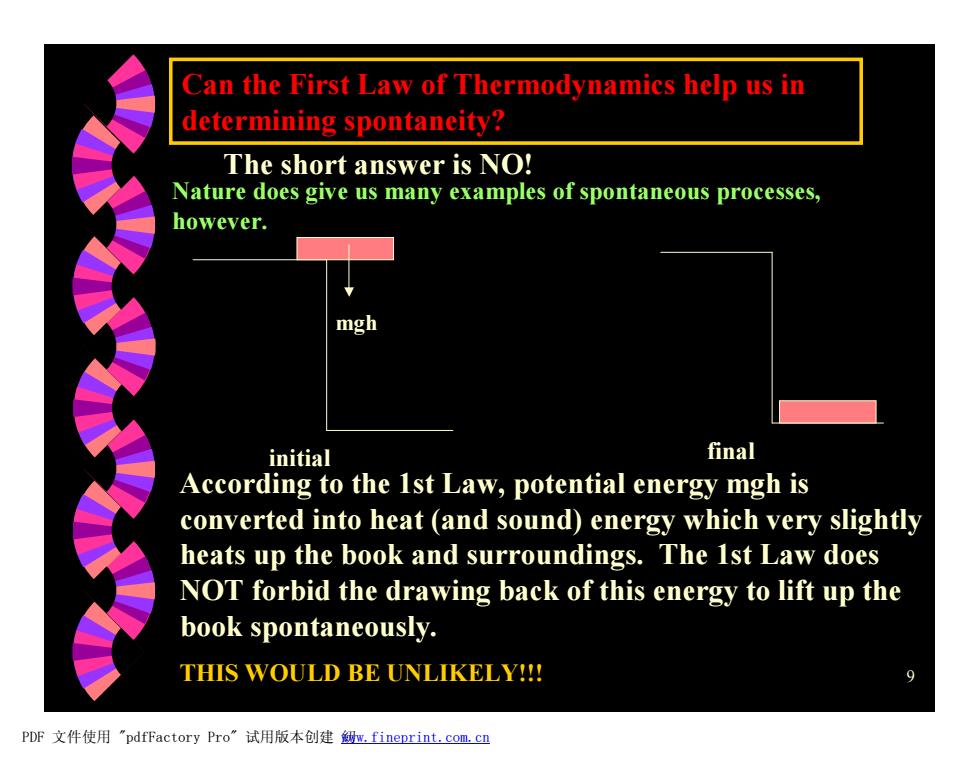
Can the First Law of Thermodynamics help us in determining spontaneity? The short answer is NO! Nature does give us many examples of spontaneous processes, however. mgh initial final According to the 1st Law,potential energy mgh is converted into heat (and sound)energy which very slightly heats up the book and surroundings.The 1st Law does NOT forbid the drawing back of this energy to lift up the book spontaneously. THIS WOULD BE UNLIKELY!!! PDF文件使用"pdfFactory Pro”试用版本创建级m,fineprint..com,c四
9 Can the First Law of Thermodynamics help us in determining spontaneity? The short answer is NO! Nature does give us many examples of spontaneous processes, however. mgh initial final According to the 1st Law, potential energy mgh is converted into heat (and sound) energy which very slightly heats up the book and surroundings. The 1st Law does NOT forbid the drawing back of this energy to lift up the book spontaneously. THIS WOULD BE UNLIKELY!!! PDF 文件使用 "pdfFactory Pro" 试用版本创建 糿www.fineprint.com.cn
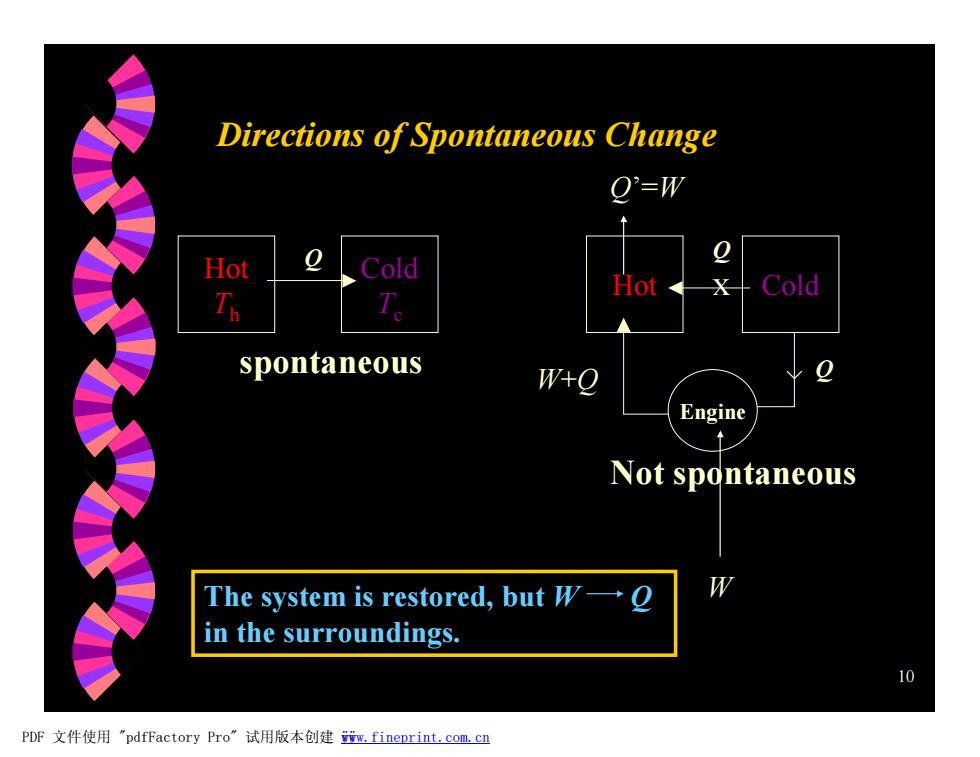
Directions of Spontaneous Change O'=w Hot Cold Hot X Cold spontaneous W+o Engine Not spontaneous The system is restored,but W- in the surroundings. 10 PDF文件使用"pdfFactory Pro” 试用版本创建m,fineprint..com,c四
10 Directions of Spontaneous Change Hot Th Cold Tc Q spontaneous Hot x Cold Q Engine Not spontaneous Q W W+Q Q’=W The system is restored, but W Q in the surroundings. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn ÿ