
Surfaces PDF文件使用"pdfFactory Pro”试用版本创建w,fineprint.com,c四
Surfaces PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì
Introduction So far,we have concentrated on the properties of the given system.However,the physical boundary between phases,such as the surface where solid is in contact with liquid or liquid is in contact with vapour, has interesting properties. In this Chapter we concentrate on the liquid-vapour interface,which is interesting because it is so mobile. and also deal with solid surfaces and their important role in catalysis. PDF文件使用"pdfFactory Pro”试用版本创建iww.fineprint.com.cn
Introduction So far, we have concentrated on the properties of the given system. However, the physical boundary between phases, such as the surface where solid is in contact with liquid or liquid is in contact with vapour, has interesting properties. In this Chapter we concentrate on the liquid-vapour interface, which is interesting because it is so mobile, and also deal with solid surfaces and their important role in catalysis. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn
Surface Tension Many properties of liquids give us direct information about the forces that exist among the particles The molecules in the interior of the droplet are surrounded by other molecules however those at the liquid surface are subject to attractions only from one side and from below The effect of this uneven oull on the surface molecules tends to draw them into the body of the droplet assuming a spherical shape -minimum surface area The resistance of a liquid to an increase in its surface area is called surface tension of the liquid. Polar solvents tend to have fairly high surface tension PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
Surface Tension b Many properties of liquids give us direct information about the forces that exist among the particles b The molecules in the interior of the droplet are surrounded by other molecules however those at the liquid surface are subject to attractions only from one side and from below b The effect of this uneven pull on the surface molecules tends to draw them into the body of the droplet assuming a spherical shape – minimum surface area b The resistance of a liquid to an increase in its surface area is called surface tension of the liquid. . Polar solvents tend to have fairly high surface tension PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn
Surface Tension Surface effects can be expressed in the language of Gibbs energy.The link between Gibbs energy and the surface area is the work needed to change the area by a given amount,and the fact that dG is equal to the work done in changing the energy of a system. The work needed to change the surface area,A,of a sample by an infinitesimal amount dA.is proportional to dA,and we write dG-ydAs Or Y=(@G/OAs)T,P,N Or Y=F/2I PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,c里
Surface Tension Surface effects can be expressed in the language of Gibbs energy.The link between Gibbs energy and the surface area is the work needed to change the area by a given amount, and the fact that dG is equal to the work done in changing the energy of a system. The work needed to change the surface area, As , of a sample by an infinitesimal amount dAs is proportional to dAs , and we write dG=γdAs Or γ=(¶G/¶As )T, P,N Or γ=F/2l PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

金属环 细线 液膜 Plane surface:the direction ofy is parallel to the plane surface. Curved surface:the direction ofy is on the tangency surface of the curved surface. PDF文件使用"pdfFactory Pro”试用版本创建截,fineprint.com,c四
Plane surface:the direction of γ is parallel to the plane surface. Curved surface: the direction of γ is on the tangency surface of the curved surface. PDF 文件使用 "pdfFactory Pro" 试用版本创建 駌ÿ www.fineprint.com.cn

The Fundamental Equation The work of forming a surface is additional to pV-work, and so we should regard it as a contribution to the Gibbs function of the system.Therefore in the presence of a system with a variable surface area dG=-SdT+VdP+Eugdng+YdAs This is the link with thermodynamics,and the reasoning about systems tending to smaller Gibbs functions can be applied to this equation.For instance,at constant T,P and N,a smaller value of the Gibbs function can be attained if the area is allowed to diminish.The condition for a natural change,dG<0,implies dA<0,which means that surfaces have a natural tendency to contract. PDF文件使用"pdfFactory Pro”试用版本创建替m.fineprint.com.cn
The Fundamental Equation The work of forming a surface is additional to pV-work, and so we should regard it as a contribution to the Gibbs function of the system. Therefore in the presence of a system with a variable surface area dG=-SdT+VdP+åmBdnB+ γdAs This is the link with thermodynamics, and the reasoning about systems tending to smaller Gibbs functions can be applied to this equation. For instance,at constant T ,P and N, a smaller value of the Gibbs function can be attained if the area is allowed to diminish. The condition for a natural change, dG<0, implies dAs <0, which means that surfaces have a natural tendency to contract. PDF 文件使用 "pdfFactory Pro" 试用版本创建 替www.fineprint.com.cn
The Effect Factors On the Surface Tension The nature of substance ·The contact phase Pressure and composition ·Temperature With the temperature increasing,usually the surface tension decreasing. PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
The Effect Factors On the Surface Tension • The nature of substance • The contact phase • Pressure and composition • Temperature With the temperature increasing, usually the surface tension decreasing. PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn
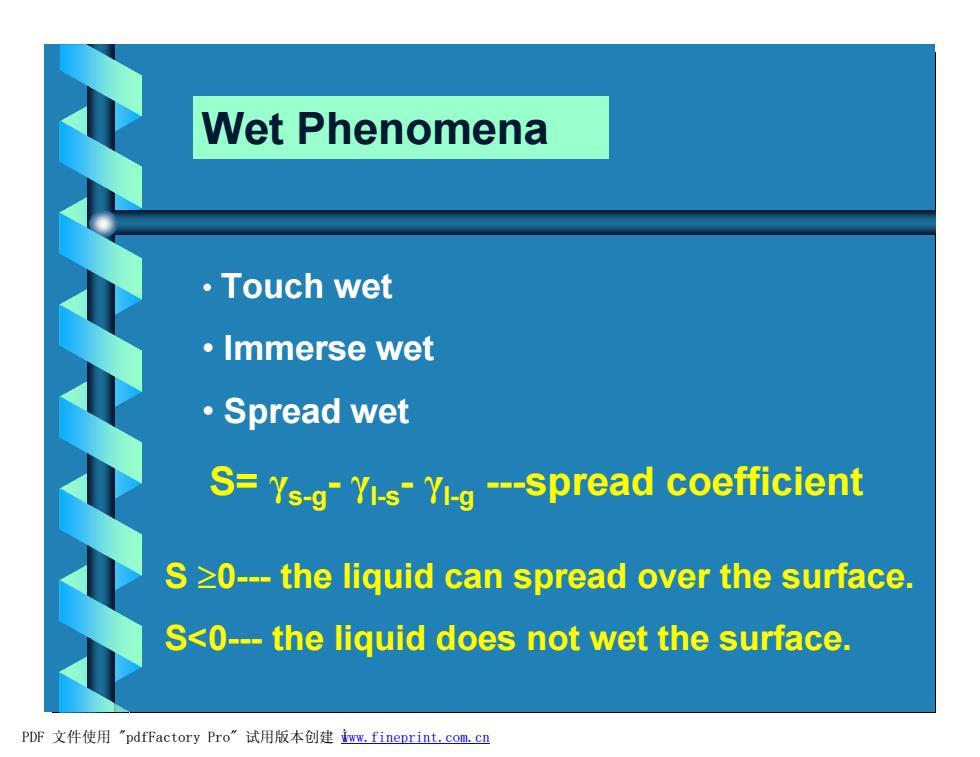
Wet Phenomena ·Touch wet ·Immerse wet ·Spread wet S=Ys-g-Yis-Y-g--spread coefficient S >0---the liquid can spread over the surface. S<0---the liquid does not wet the surface. PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,c四
Wet Phenomena • Touch wet • Immerse wet • Spread wet S= γs-g- γl-s- γl-g ---spread coefficient S ³0--- the liquid can spread over the surface. S<0--- the liquid does not wet the surface. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn
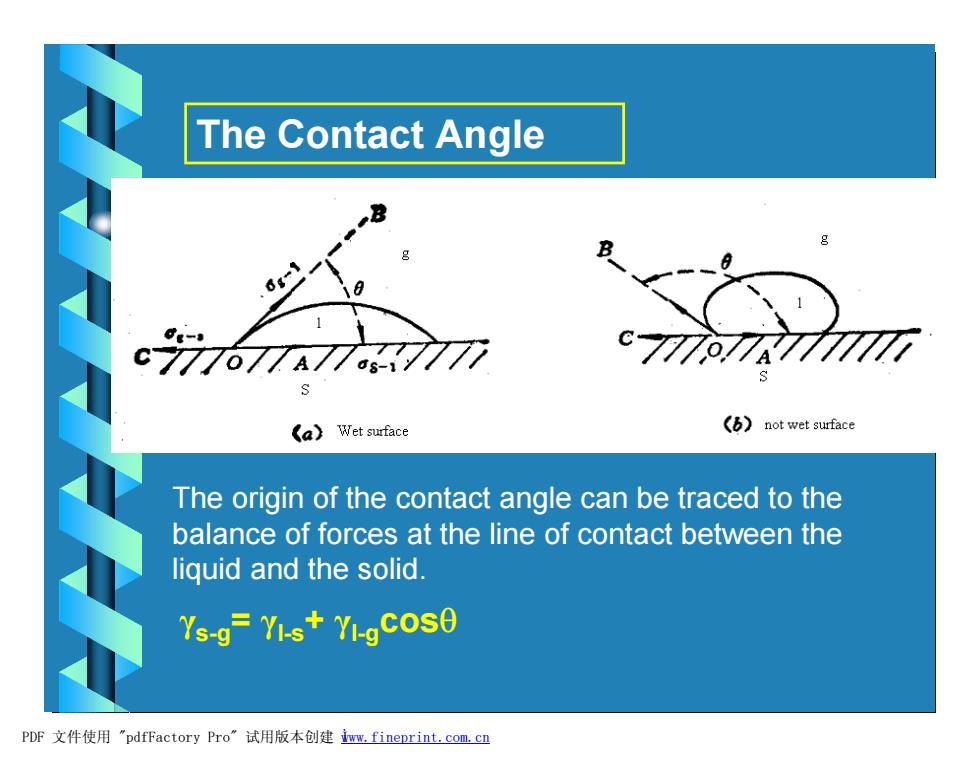
The Contact Angle - c777o77A 77 ds-1 《a)Wet surface (not wet surface The origin of the contact angle can be traced to the balance of forces at the line of contact between the liquid and the solid. Ys-g=YI-s+YI-gCOSO PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
The Contact Angle The origin of the contact angle can be traced to the balance of forces at the line of contact between the liquid and the solid. γs-g= γl-s+ γl-gcosq PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

Discussion: 0<0<90---the liquid wets the surface 90°<0<180°--the liquid does not wet the surface. PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint,com,cn
Discussion: • 0< q<90°---the liquid wets the surface •90°< q<180°---the liquid does not wet the surface. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn