正在加载图片...
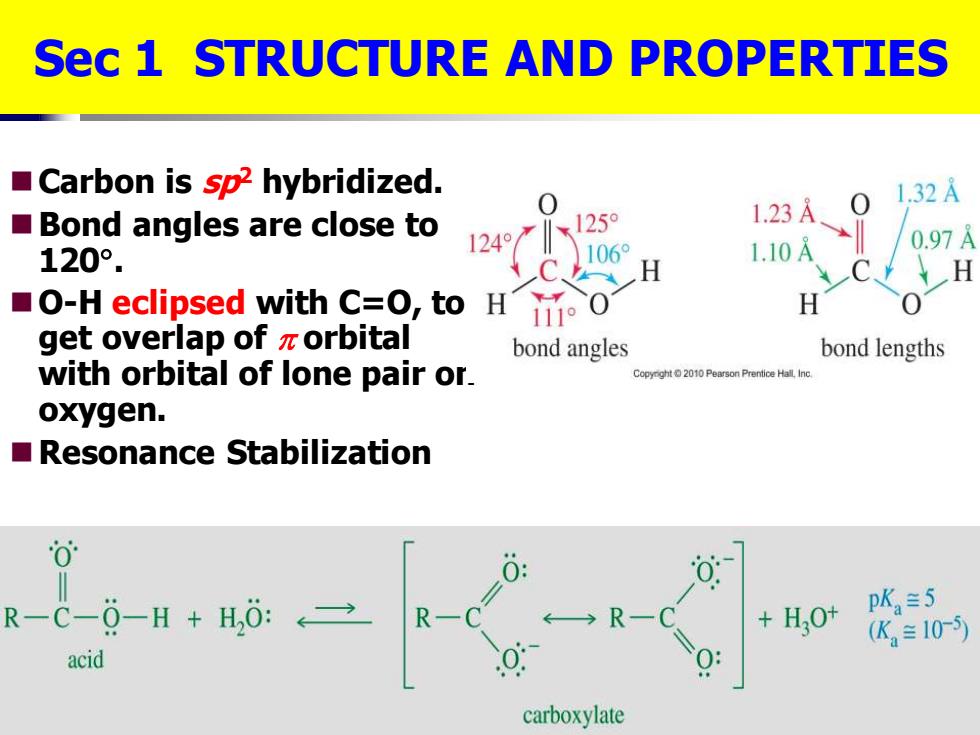
Sec 1 STRUCTURE AND PROPERTIES Carbon is sp2 hybridized. 1247X12 0 1.32A Bond angles are close to 1.23A 120°. 1060 1.10A 0.97 H O-H eclipsed with C=O,to H 111°0 H get overlap of zorbital bond angles bond lengths with orbital of lone pair or. Copyght2010 Pearson Prentice Hall Inc oxygen. Resonance Stabilization 0 R-C-0-H H,0: +H,0t pK,=5 R- (K≡10-5) acid carboxylate ◼Carbon is sp2 hybridized. ◼Bond angles are close to 120 . ◼O-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. ◼Resonance Stabilization Sec 1 STRUCTURE AND PROPERTIES