正在加载图片...
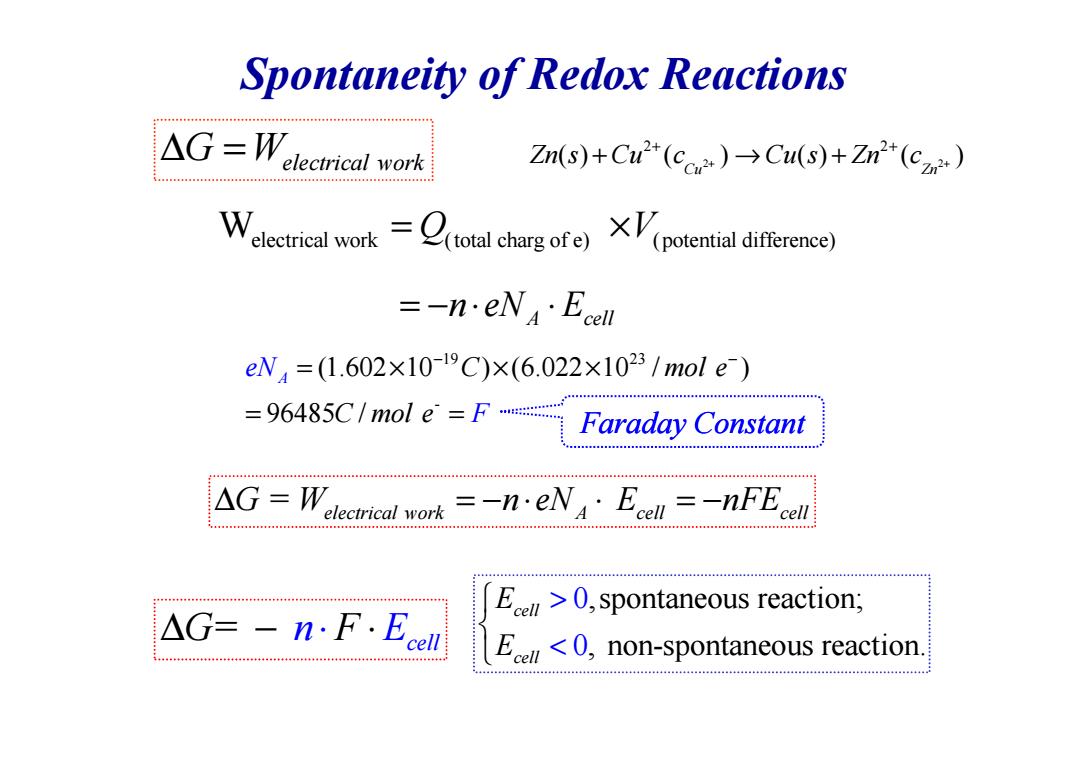
Spontaneity of Redox Reactions △G=W.lecricalrk Zn(s)+Cu"(c)Cu(s)+Zn(c) Welectrical worktotal charg of)(ptential diferen) =-n.eNEcel eW4=(1.602×10-19C)×(6.022×1023/mole) =96485C/mol e=F Faraday Constant △G=W elecmrical work =neNA Ecell =-nFE cell △G=-n:F.Eel E>0,spontaneous reaction; E<0,non-spontaneous reaction.Spontaneity of Redox Reactions W electrical work (total charg of e) (potent = × Q V ial difference) A cell = − ⋅ ⋅ n eN E 19 23 (1.602 10 ) (6.022 10 / ) A eN C mol e − − = × × × 2 2 2 2 ( ) ( ) ( ) ( ) Cu Zn Zn s Cu c Cu s Zn c + + + + ∆ = G Welectrical work + → + - (1.602 10 ) (6.022 10 / ) 96485 / A eN F C mol e C mol e = × × × = = = ∆ = − ⋅ ⋅ = − G W n eN E nFE electrical work A cell cell = G cell ∆ − ⋅ n⋅F E ,spontaneous reaction; , non-spontaneous reaction. 0 0 cell cell E E > < Faraday Constant