正在加载图片...
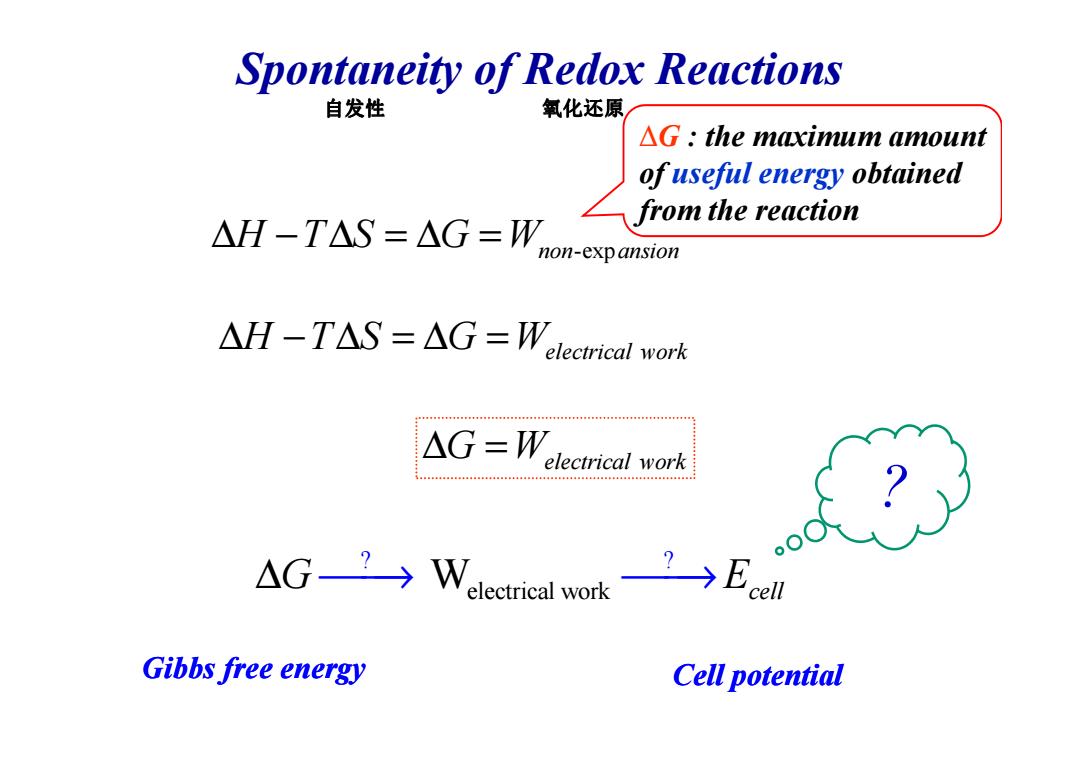
Spontaneity of Redox Reactions 自发性 氧化还原 △G:the maximum amount of useful energy obtained AH-TAS=△G=W乙from the reaction non-exp ansion △H-T△S=△G=Welectricalork △G=Welecrical work Gibbs free energy Cell potentialSpontaneity of Redox Reactions ∆ − ∆ = ∆ = H T S G Wnon ansion -exp ∆G : the maximum amount of useful energy obtained from the reaction ∆ − ∆ = ∆ = H T S G Welectrical work 自发性 氧化还原 Gibbs free energy electrical work ? ? ∆G E → → W cell ∆ = G Welectrical work ? ? Cell potential