正在加载图片...
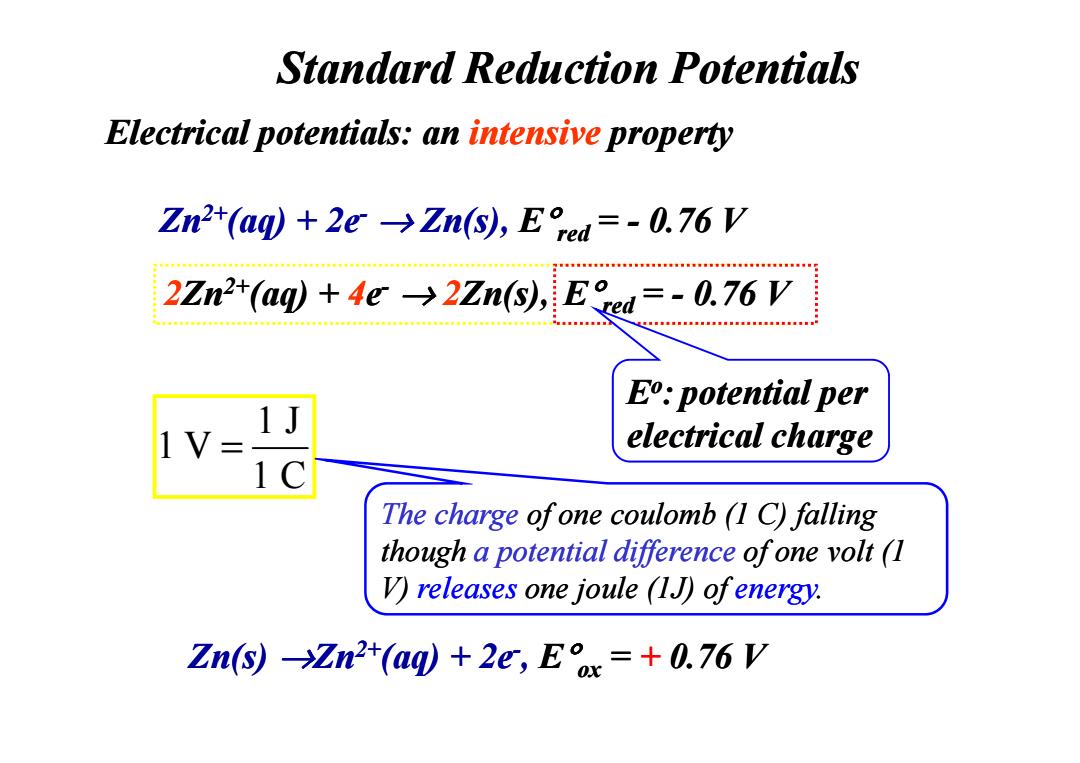
Standard Reduction Potentials Electrical potentials:an intensive property Zn2+(aq)+2e->Zn(s),Eed=-0.76 V 2Zn2+(aq)+4e->2Zn(s),E ed=-0.76 V E:potential per electrical charge The charge ofone coulomb (1 C)falling though a potential difference ofone volt (1 V)releases one joule (1J)ofenergy. Zn(s)Zn2t(aq)+2e,Ex=+0.76 VZn2+(aq) + 2e- → Zn(s), E°red = - 0.76 V Standard Reduction Potentials Electrical potentials: an intensive property 2Zn2+(aq) + 4e- → 2Zn(s), E°E°red red= ? V = - 0.76 V Eo: potential per electrical charge Zn(s) →Zn2+(aq) + 2e-, E°ox = + 0.76 V 1 J 1 V 1 C = The charge of one coulomb (1 C) falling though a potential difference of one volt (1 V) releases one joule (1J) of energy