正在加载图片...
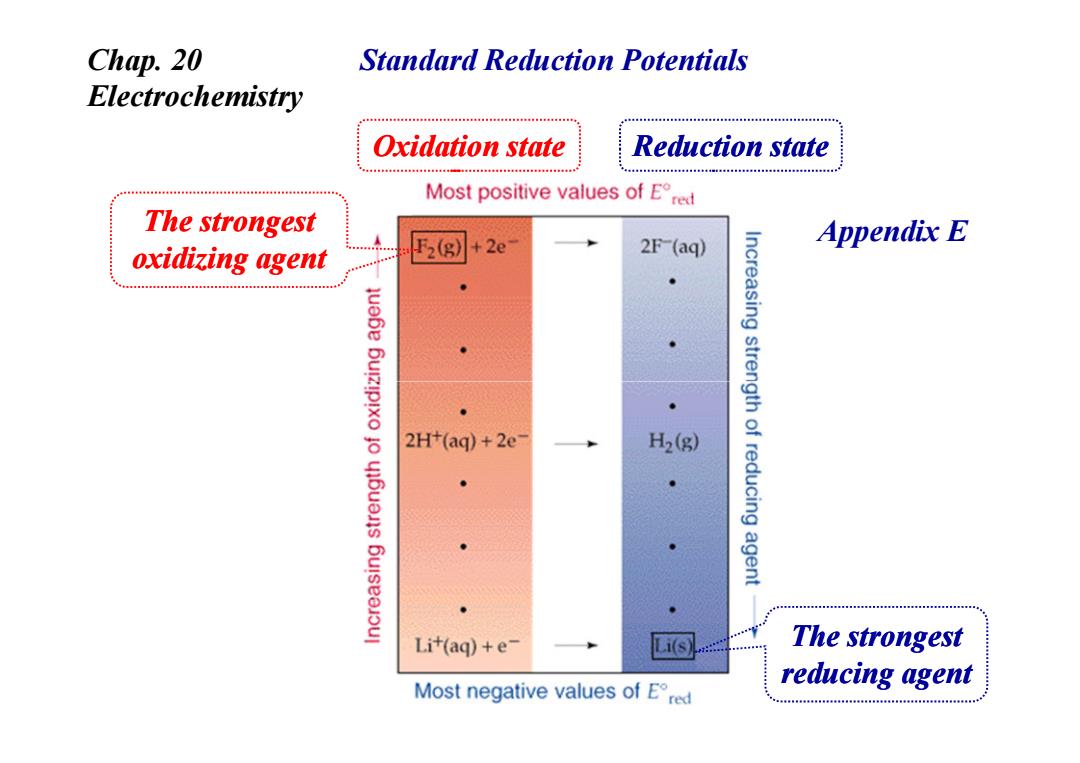
Chap.20 Standard Reduction Potentials Electrochemistry Oxidation state Reduction state Most positive values of Ere The strongest 2e Appendix E oxidizing agent 2(g 2F-(aq) 2H+(aq)+2e- H2(g) Increasing strength of reducing agent Li+(aq)+e- Li(s The strongest Most negative values of Ered reducing agentStandard Reduction Potentials Oxidation state Reduction state Chap. 20 Electrochemistry The strongest oxidizing agent Appendix E The strongest reducing agent