正在加载图片...
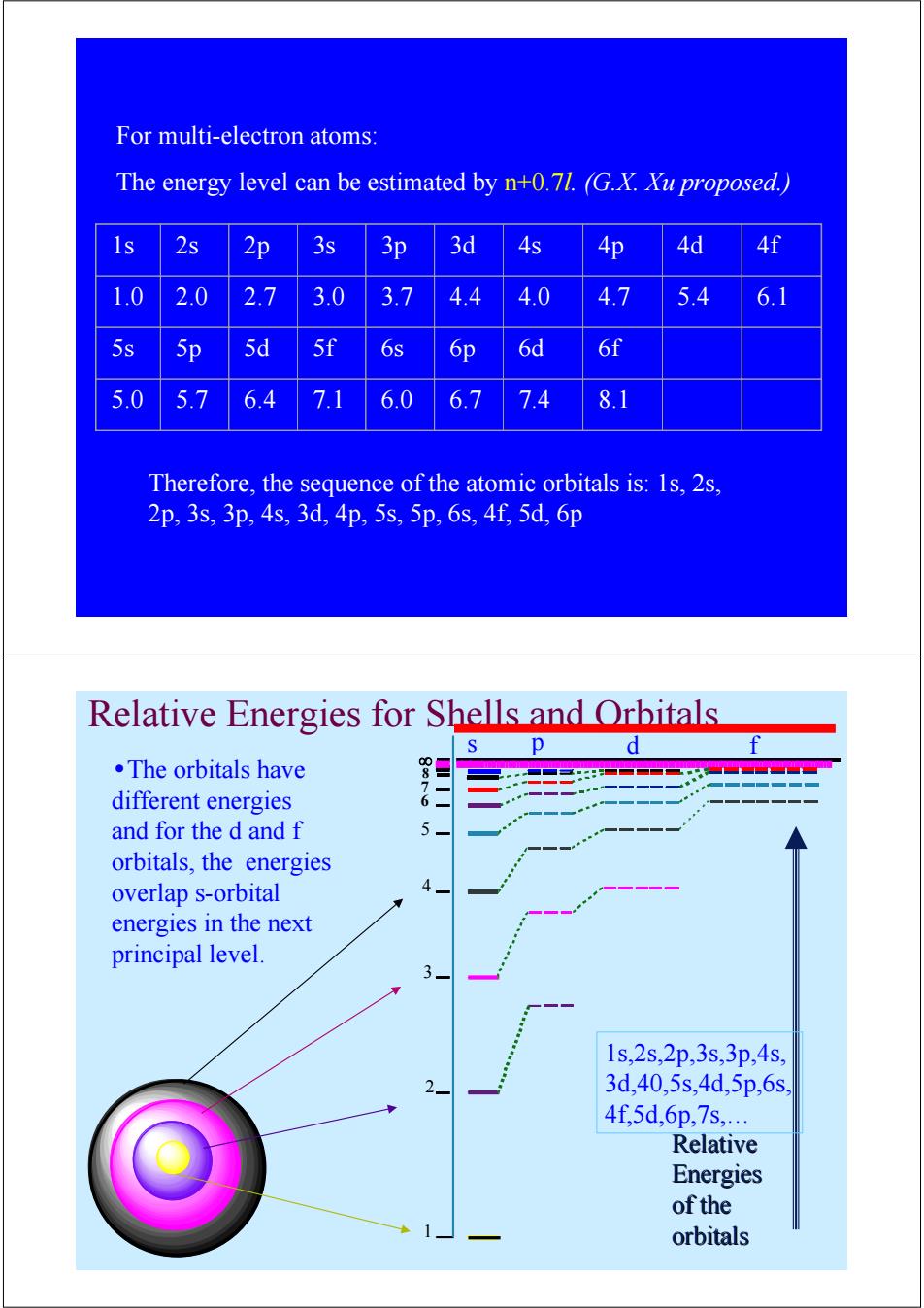
For multi-electron atoms: The energy level can be estimated by n+0.71.(G.X.Xu proposed.) ls 2s 2p 3s 3p 3d 4s 4p 4d 4f 1.0 2.0 2.7 3.0 3.7 4.4 4.0 4.7 5.4 6.1 5s 5p 5d 5f 6s 6p 6d 6f 5.0 5.7 6.4 7.1 6.0 6.7 7.4 8.1 Therefore,the sequence of the atomic orbitals is:1s,2s, 2p,3s,3p,4s,3d,4p,5s,5p,6s,4f5d,6p Relative Energies for Shells and Orbitals p d f ·The orbitals have different energies and for the d and f orbitals,the energies overlap s-orbital energies in the next principal level. 1s,2s,2p,3s,3p,4s 3d,40,5s,4d,5p,6s, 4f,5d,6p,7s, Relative Energies of the orbitalsFor multi-electron atoms: The energy level can be estimated by n+0.7l. (G.X. Xu proposed.) 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 1.0 2.0 2.7 3.0 3.7 4.4 4.0 4.7 5.4 6.1 5s 5p 5d 5f 6s 6p 6d 6f 5.0 5.7 6.4 7.1 6.0 6.7 7.4 8.1 Therefore, the sequence of the atomic orbitals is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 5p, 6s, 4f, 5d, 6p Relative Energies for Shells and Orbitals •The orbitals have different energies and for the d and f orbitals, the energies overlap s-orbital energies in the next principal level. 1 2 3 4 5 6 7 ∞ 8 s p d f Relative Relative Energies Energies of the orbitals orbitals 1s,2s,2p,3s,3p,4s, 3d,40,5s,4d,5p,6s, 4f,5d,6p,7s,…