正在加载图片...
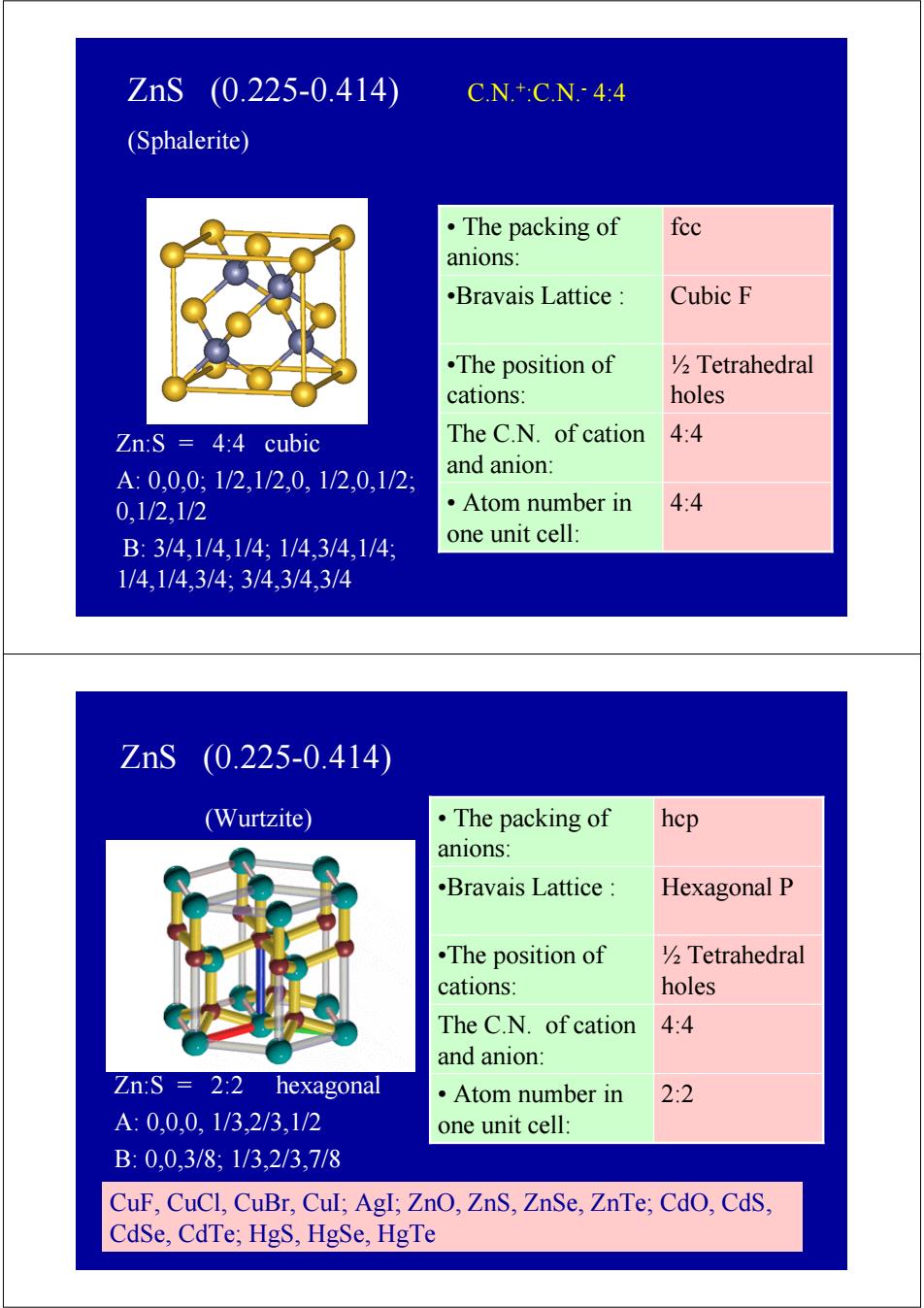
ZnS (0.225-0.414) CN+CN.-4:4 (Sphalerite) ·The packing of fec anions .Bravais Lattice Cubic F •The position of V2 Tetrahedral cations: holes Zn:S =4:4 cubic The C.N.of cation 4:4 and anion: A:0,0,0,1/2,1/2,0,1/2,0,1/2 0,1/2,1/2 ·Atom number in 4:4 B:3/4,1/4,1/4:1/4,3/4,1/4: one unit cell: 1/4,1/4,3/4;3/4,3/4,3/4 ZnS (0.225-0.414 (Wurtzite) ·The packing of hcp anions: .Bravais Lattice Hexagonal P .The position of 2 Tetrahedral cations: holes The C.N.of cation 4:4 and anion: Zn:S 2:2 hexagonal ·Atom number in 2:2 A:0,0,0,1/3,2/3,1/2 one unit cell: B:0,0,3/8,1/3,2/3,7/8 CuF,CuCl,CuBr,Cul;AgI;ZnO,ZnS,ZnSe,ZnTe;CdO,CdS, CdSe,CdTe;HgS,HgSe,HgTeZnS (0.225-0.414) C.N.+:C.N.- 4:4 (Sphalerite) Zn:S = 4:4 cubic A: 0,0,0; 1/2,1/2,0, 1/2,0,1/2; 0,1/2,1/2 B: 3/4,1/4,1/4; 1/4,3/4,1/4; 1/4,1/4,3/4; 3/4,3/4,3/4 •Bravais Lattice : Cubic F • Atom number in 4:4 one unit cell: The C.N. of cation 4:4 and anion: ½ Tetrahedral holes •The position of cations: • The packing of fcc anions: Zn:S = 2:2 hexagonal A: 0,0,0, 1/3,2/3,1/2 B: 0,0,3/8; 1/3,2/3,7/8 (Wurtzite) ZnS (0.225-0.414) CuF, CuCl, CuBr, CuI; AgI; ZnO, ZnS, ZnSe, ZnTe; CdO, CdS, CdSe, CdTe; HgS, HgSe, HgTe •Bravais Lattice : Hexagonal P • Atom number in 2:2 one unit cell: The C.N. of cation 4:4 and anion: ½ Tetrahedral holes •The position of cations: • The packing of hcp anions: