正在加载图片...
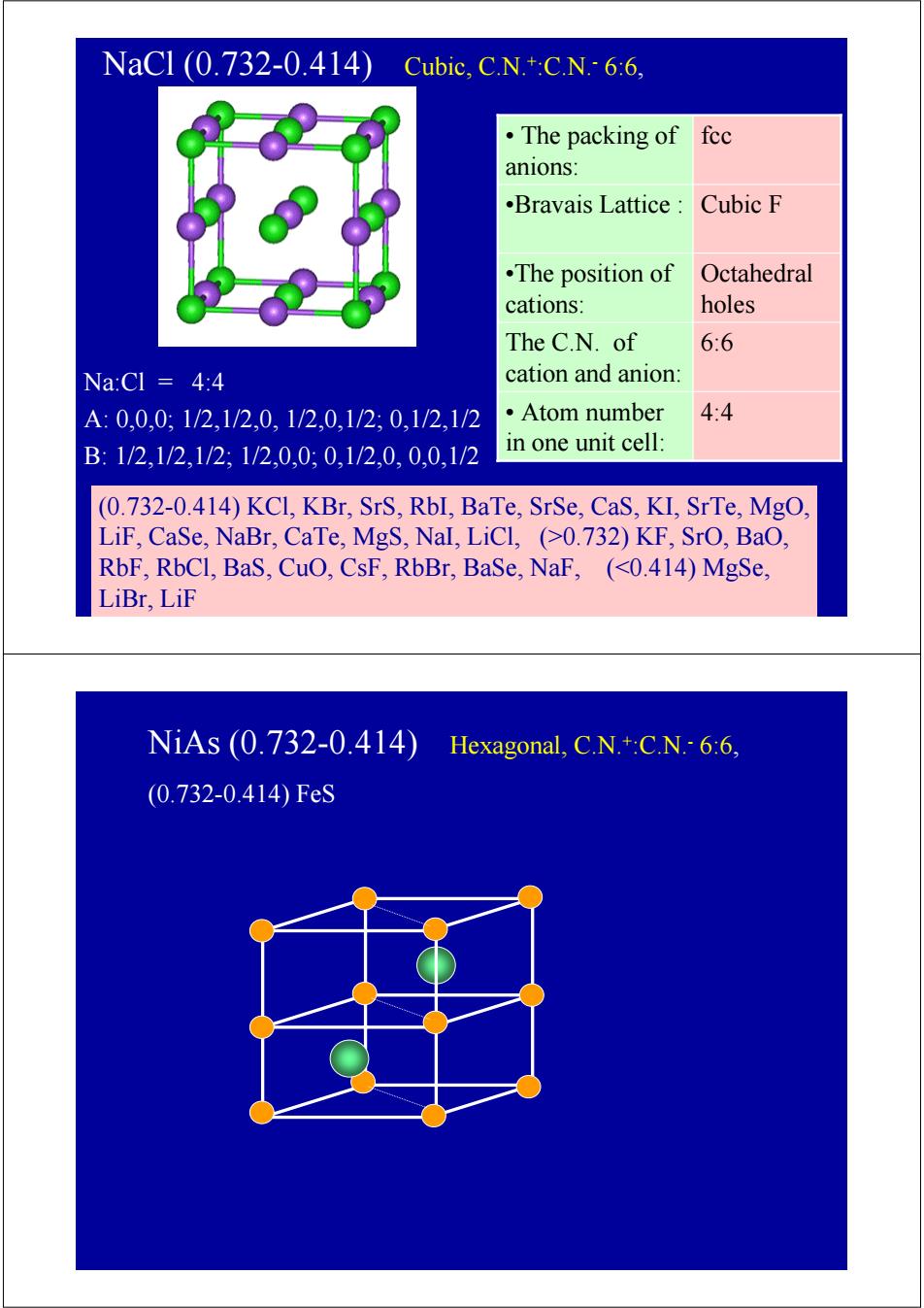
NaCl(0.732-0.414)Cubic,.C.N+c.N-66, ·The packing of fec anions .Bravais Lattice Cubic F .The position of Octahedral cations: holes The C.N.of 6:6 Na:Cl 4:4 cation and anion: A:0,0,0;1/2,1/2,0,1/2,0,1/2,0,1/2,1/2 ·Atom number 4:4 B:1/2,1/2,1/2;1/2,0,0:0,1/2,0,0,0,1/2 in one unit cell: (0.732-0.414)KCl,KBr,SrS,RbI,BaTe,SrSe,CaS,KI,SrTe,MgO, LiF,CaSe,NaBr,CaTe,MgS,NaI,LiCl,(>0.732)KF,SrO,BaO, RbF,RbCl,BaS,CuO,CsF,RbBr,BaSe,NaF,(<0.414)MgSe, LiBr,LiF NiAs(0.732-0.414) Hexagonal,C.N.+:C.N.6:6, (0.732-0.414)FeSNaCl (0.732-0.414) Cubic, C.N.+:C.N.- 6:6, Na:Cl = 4:4 A: 0,0,0; 1/2,1/2,0, 1/2,0,1/2; 0,1/2,1/2 B: 1/2,1/2,1/2; 1/2,0,0; 0,1/2,0, 0,0,1/2 (0.732-0.414) KCl, KBr, SrS, RbI, BaTe, SrSe, CaS, KI, SrTe, MgO, LiF, CaSe, NaBr, CaTe, MgS, NaI, LiCl, (>0.732) KF, SrO, BaO, RbF, RbCl, BaS, CuO, CsF, RbBr, BaSe, NaF, (<0.414) MgSe, LiBr, LiF •Bravais Lattice : Cubic F • Atom number 4:4 in one unit cell: The C.N. of 6:6 cation and anion: Octahedral holes •The position of cations: • The packing of fcc anions: NiAs (0.732-0.414) Hexagonal, C.N.+:C.N.- 6:6, (0.732-0.414) FeS