正在加载图片...
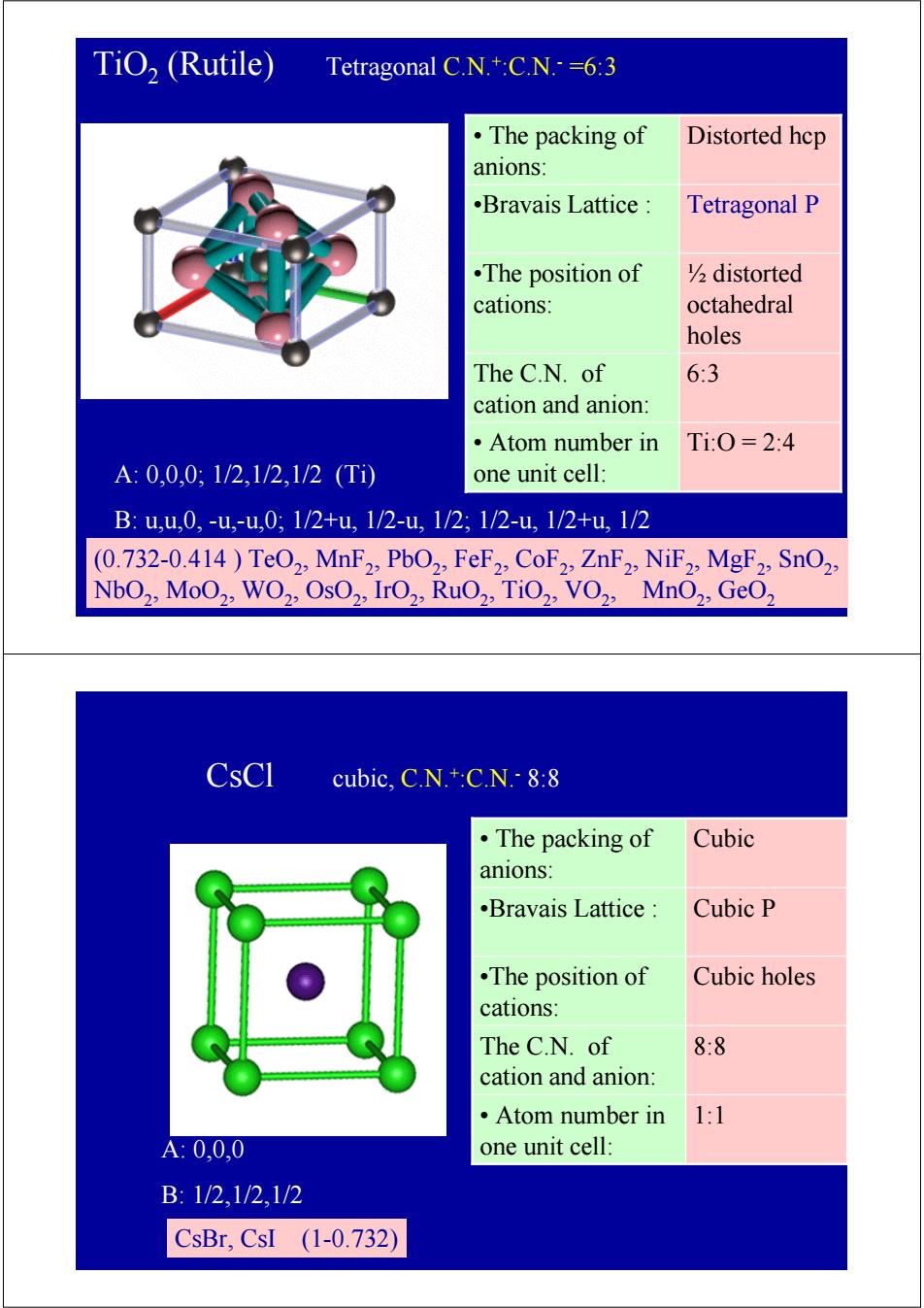
TiO,(Rutile) Tetragonal C.N.+:C.N.=6:3 ·The packing of Distorted hep anions: .Bravais Lattice: Tetragonal P •The position of V2distorted cations: octahedral holes The C.N.of 6:3 cation and anion: ·Atom number in Ti:0=2:4 A:0,0,0:1/2,1/2,1/2(T1 one unit cell: B:u,u,0,-u,-u,0;1/2+u,1/2-u,1/2;1/2-u,1/2+u,1/2 (0.732-0.414)TeO2,MnF2,PbO2,FeF2,CoF2,ZnF2,NiF2,MgF2:SnO2, NbO2,MoO2,WO2,OsO2,IrO2,RuO2,TiO2,VO2,MnO2,GeO2 CsCl cubic,C.N.+:C.N.-8:8 ·The packing of Cubic anions: .Bravais Lattice Cubic P .The position of Cubic holes cations: The C.N.of 8:8 cation and anion: ·Atom number in 1:1 A:0,0,0 one unit cell: B:1/2,1/2,1/2 CsBr,CsI (1-0.732)TiO2 (Rutile) Tetragonal C.N.+:C.N.- =6:3 A: 0,0,0; 1/2,1/2,1/2 (Ti) B: u,u,0, -u,-u,0; 1/2+u, 1/2-u, 1/2; 1/2-u, 1/2+u, 1/2 (0.732-0.414 ) TeO2, MnF2, PbO2, FeF2, CoF2, ZnF2, NiF2, MgF2, SnO2, NbO2, MoO2, WO2, OsO2, IrO2, RuO2, TiO2, VO2, MnO2, GeO2 •Bravais Lattice : Tetragonal P • Atom number in Ti:O = 2:4 one unit cell: The C.N. of 6:3 cation and anion: ½ distorted octahedral holes •The position of cations: • The packing of Distorted hcp anions: CsCl cubic, C.N.+:C.N.- 8:8 A: 0,0,0 B: 1/2,1/2,1/2 CsBr, CsI (1-0.732) •Bravais Lattice : Cubic P • Atom number in 1:1 one unit cell: The C.N. of 8:8 cation and anion: •The position of Cubic holes cations: • The packing of Cubic anions: