正在加载图片...
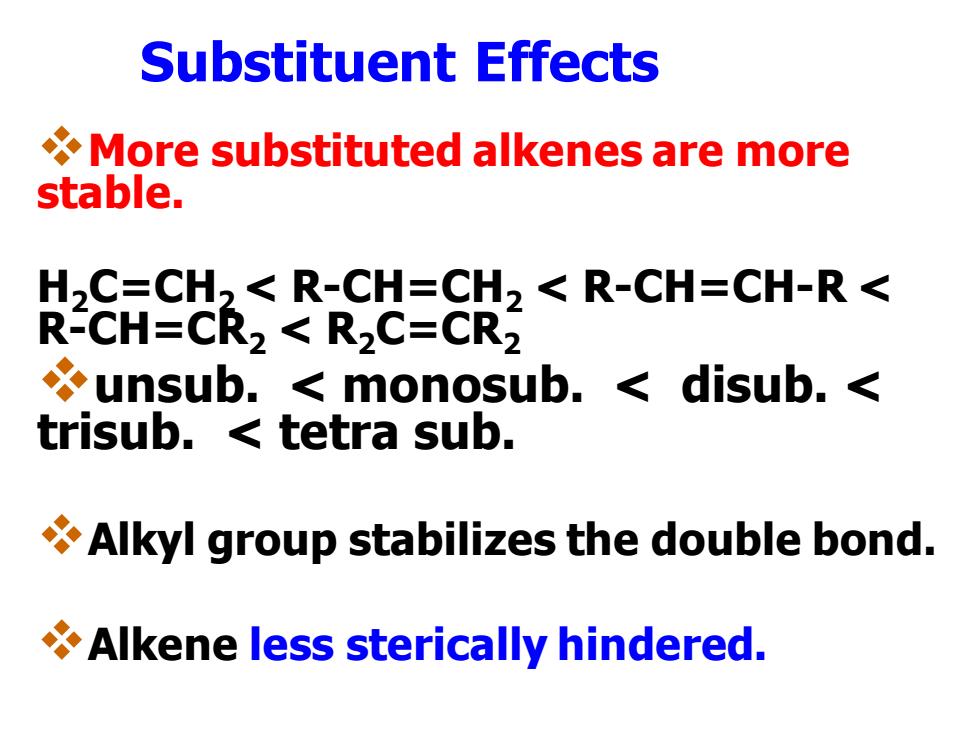
Substituent Effects More substituted alkenes are more stable. H,C=CH2<R-CH=CH2<R-CH=CH-R< R-CH=CR2 R2C=CR2 unsub.monosub.disub.< trisub.tetra sub. Alkyl group stabilizes the double bond. Alkene less sterically hindered. Substituent Effects ❖More substituted alkenes are more stable. H2C=CH2 < R-CH=CH2 < R-CH=CH-R < R-CH=CR2 < R2C=CR2 ❖unsub. < monosub. < disub. < trisub. < tetra sub. ❖Alkyl group stabilizes the double bond. ❖Alkene less sterically hindered