正在加载图片...
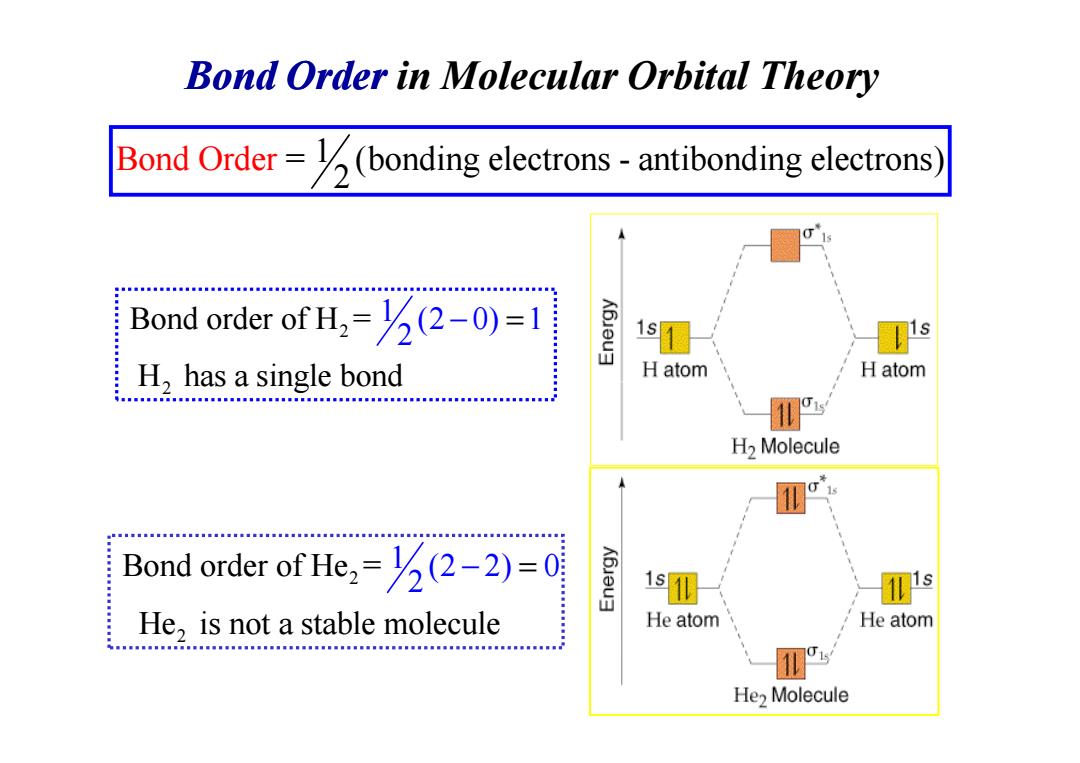
Bond Order in Molecular Orbital Theory Bond Order=(bonding electrons-antibonding electrons Bond order of H=(-0)=1 1s H,has a single bond H atom H atom CHHH.HEHBH....MNNAM.MNN.MM3n...l... H2 Molecule Bond order of He=(2-2)=0 He,is not a stable molecule He atom He atom He>MoleculeBond Order in Molecular Orbital Theory Bond Ord = (bonding electrons - antibonding 1 electr 2 er ons) 2 2 1 Bond order of H = (2 0) 1 H has a singl 2 e bond − = 2 H has a singl 2 2 Bond order of He = He is not a stable 1 (2 m 2) 0 2 olecule − =