正在加载图片...
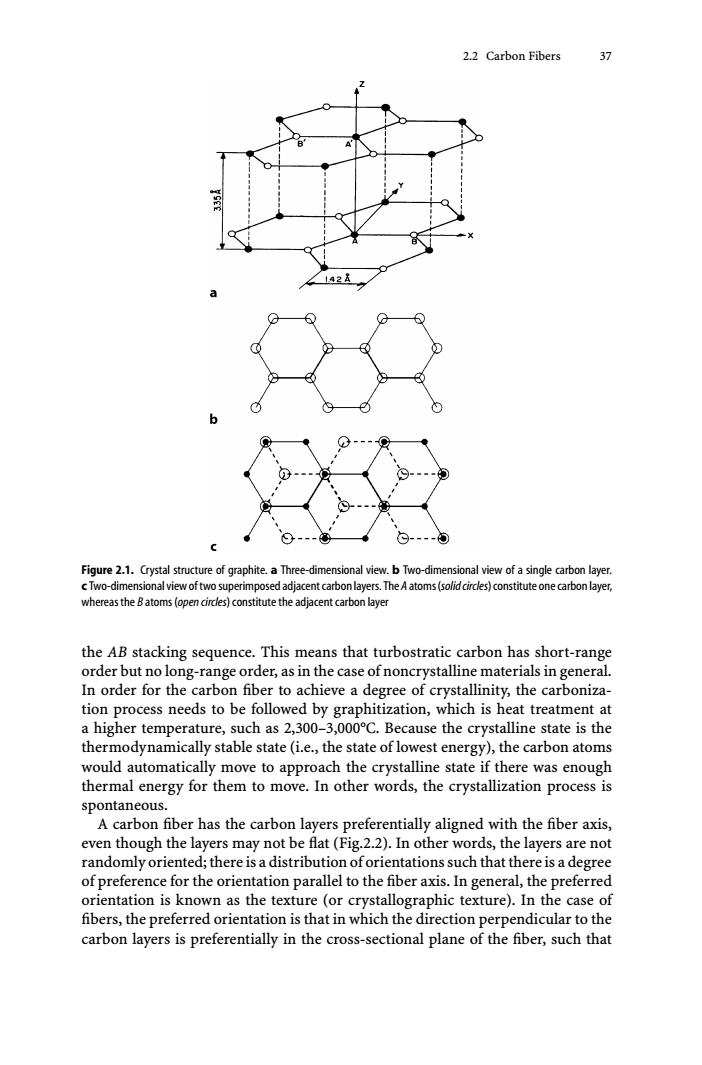
2.2 Carbon Fibers 37 Figure 2.1.Crystal structure of graphite.a Three-dimensional view.b Two-dimensional view of a single carbon layer. cTwo-dimensional view of two superimposed adjacent carbon layers.The A atoms(solid circles)constitute one carbon layer, whereas the Batoms(open circles)constitute the adjacent carbon layer the AB stacking sequence.This means that turbostratic carbon has short-range order but no long-range order,as in the case of noncrystalline materials in general. In order for the carbon fiber to achieve a degree of crystallinity,the carboniza- tion process needs to be followed by graphitization,which is heat treatment at a higher temperature,such as 2,300-3,000C.Because the crystalline state is the thermodynamically stable state(i.e.,the state of lowest energy),the carbon atoms would automatically move to approach the crystalline state if there was enough thermal energy for them to move.In other words,the crystallization process is spontaneous. A carbon fiber has the carbon layers preferentially aligned with the fiber axis, even though the layers may not be flat (Fig.2.2).In other words,the layers are not randomly oriented;there is a distribution oforientations such that there is a degree of preference for the orientation parallel to the fiber axis.In general,the preferred orientation is known as the texture (or crystallographic texture).In the case of fibers,the preferred orientation is that in which the direction perpendicular to the carbon layers is preferentially in the cross-sectional plane of the fiber,such that2.2 Carbon Fibers 37 Figure 2.1. Crystal structure of graphite. a Three-dimensional view. b Two-dimensional view of a single carbon layer. c Two-dimensionalviewoftwosuperimposedadjacentcarbonlayers.The A atoms(solidcircles)constituteonecarbonlayer, whereas the B atoms (open circles) constitute the adjacent carbon layer the AB stacking sequence. This means that turbostratic carbon has short-range order but no long-range order, as in the case of noncrystalline materials in general. In order for the carbon fiber to achieve a degree of crystallinity, the carbonization process needs to be followed by graphitization, which is heat treatment at a higher temperature, such as 2,300–3,000°C. Because the crystalline state is the thermodynamically stable state (i.e., the state of lowest energy), the carbon atoms would automatically move to approach the crystalline state if there was enough thermal energy for them to move. In other words, the crystallization process is spontaneous. A carbon fiber has the carbon layers preferentially aligned with the fiber axis, even though the layers may not be flat (Fig.2.2). In other words, the layers are not randomly oriented; there is a distribution of orientations such that there is a degree of preference for the orientation parallel to the fiber axis. In general, the preferred orientation is known as the texture (or crystallographic texture). In the case of fibers, the preferred orientation is that in which the direction perpendicular to the carbon layers is preferentially in the cross-sectional plane of the fiber, such that