正在加载图片...
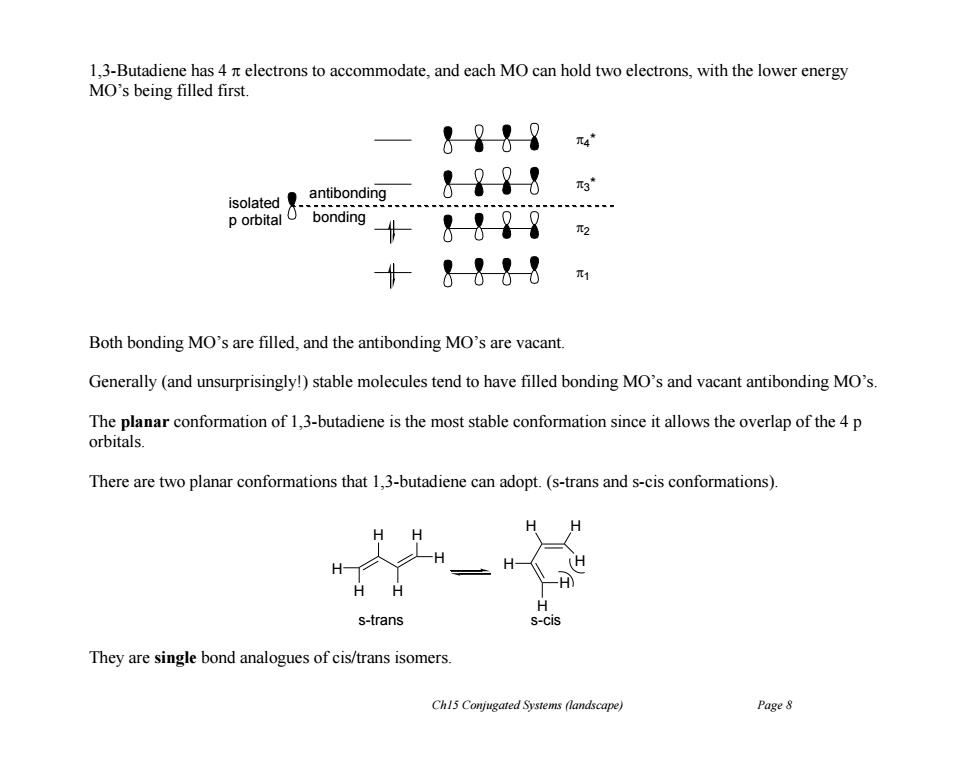
1,3-Butadiene has 4 n electrons to accommodate,and each MO can hold two electrons,with the lower energy MO's being filled first. &?↓9 antibonding &??8 isolated具. p orbital O bonding &-98 十&1-88m Both bonding MO's are filled,and the antibonding MO's are vacant Generally (and unsurprisingly!)stable molecules tend to have filled bonding MO's and vacant antibonding MO's. The planar conformation of 1,3-butadiene is the most stable conformation since it allows the overlap of the 4 p orbitals. There are two planar conformations that 1,3-butadiene can adopt.(s-trans and s-cis conformations). H (H H s-trans S-Cis They are single bond analogues of cis/trans isomers. Ch15 Conjugated Systems (landscape) Page 8Ch15 Conjugated Systems (landscape) Page 8 1,3-Butadiene has 4 electrons to accommodate, and each MO can hold two electrons, with the lower energy MO’s being filled first. Both bonding MO’s are filled, and the antibonding MO’s are vacant. Generally (and unsurprisingly!) stable molecules tend to have filled bonding MO’s and vacant antibonding MO’s. The planar conformation of 1,3-butadiene is the most stable conformation since it allows the overlap of the 4 p orbitals. There are two planar conformations that 1,3-butadiene can adopt. (s-trans and s-cis conformations). They are single bond analogues of cis/trans isomers. 4 * 3 * 2 1 isolated p orbital antibonding bonding H H H H H H H H H H H H s-trans s-cis