正在加载图片...
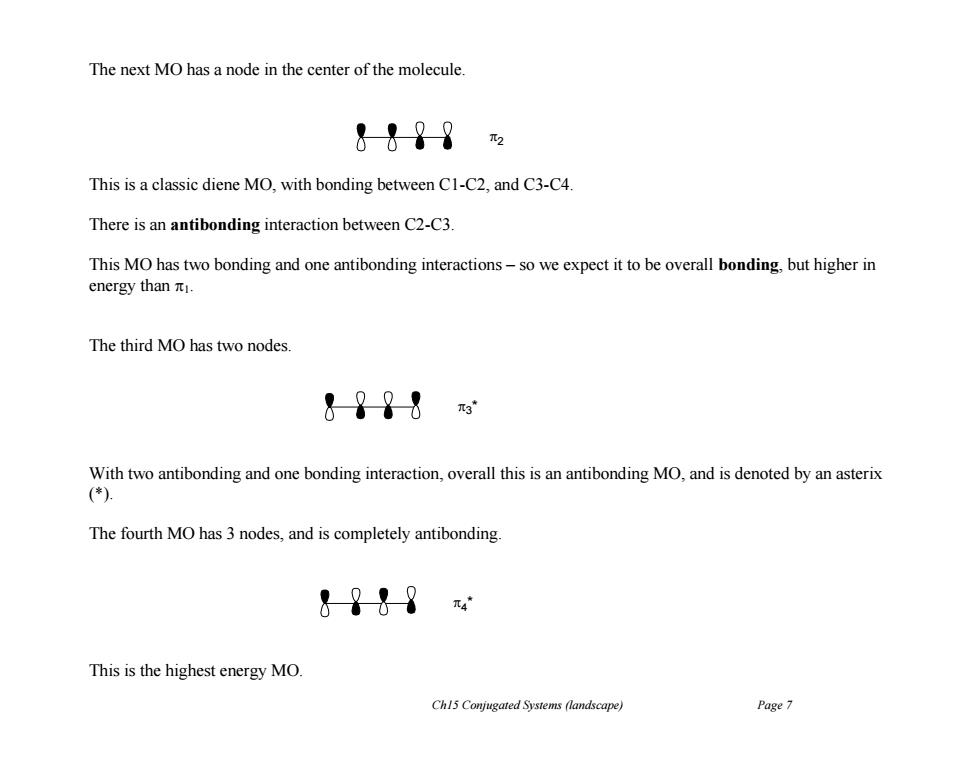
The next MO has a node in the center of the molecule. &&?8 This is a classic diene MO,with bonding between C1-C2,and C3-C4. There is an antibonding interaction between C2-C3. This MO has two bonding and one antibonding interactions-so we expect it to be overall bonding,but higher in energy than 1. The third MO has two nodes. &?98 With two antibonding and one bonding interaction,overall this is an antibonding MO,and is denoted by an asterix (*) The fourth MO has 3 nodes,and is completely antibonding &9t9 元4 This is the highest energy MO. Ch15 Conjugated Systems (landscape) Page 7 Ch15 Conjugated Systems (landscape) Page 7 The next MO has a node in the center of the molecule. This is a classic diene MO, with bonding between C1-C2, and C3-C4. There is an antibonding interaction between C2-C3. This MO has two bonding and one antibonding interactions – so we expect it to be overall bonding, but higher in energy than 1. The third MO has two nodes. With two antibonding and one bonding interaction, overall this is an antibonding MO, and is denoted by an asterix (*). The fourth MO has 3 nodes, and is completely antibonding. This is the highest energy MO. 2 3 * 4 *