正在加载图片...
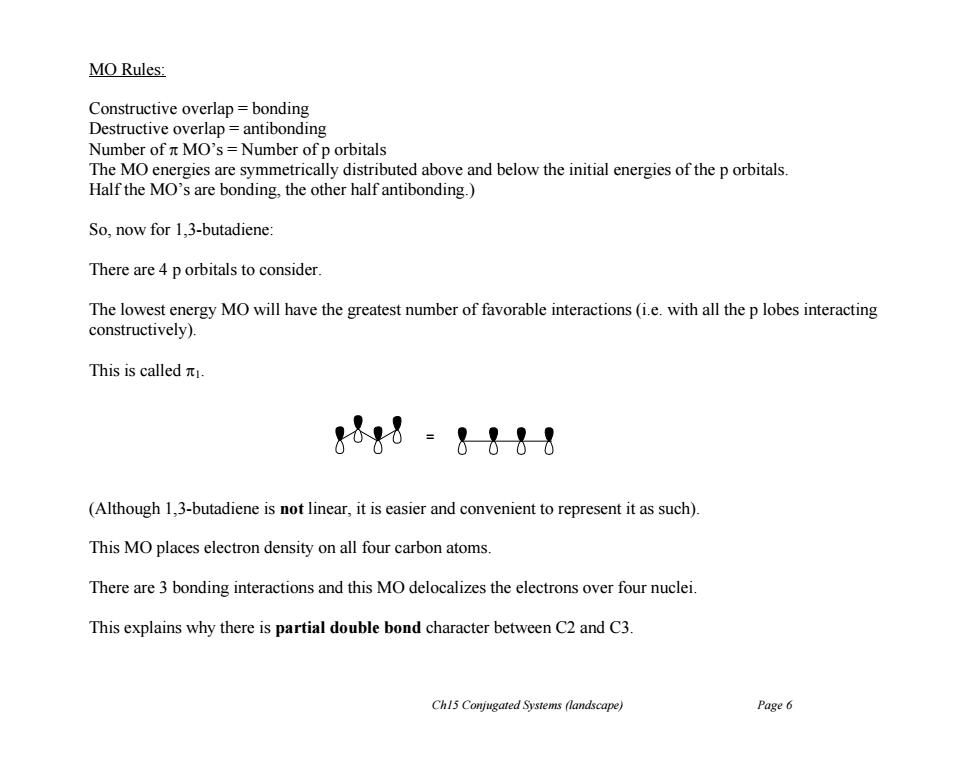
MO Rules: Constructive overlap bonding Destructive overlap=antibonding Number of n MO's=Number of p orbitals The MO energies are symmetrically distributed above and below the initial energies of the p orbitals Half the MO's are bonding,the other half antibonding.) So,now for 1.3-butadiene: There are 4 p orbitals to consider. The lowest energy MO will have the greatest number of favorable interactions (i.e.with all the p lobes interacting constructively). This is calledπ. &1&8 (Although 1,3-butadiene is not linear,it is easier and convenient to represent it as such). This MO places electron density on all four carbon atoms. There are 3 bonding interactions and this MO delocalizes the electrons over four nuclei. This explains why there is partial double bond character between C2 and C3. Ch15 Conjugated Systems (landscape) Page 6Ch15 Conjugated Systems (landscape) Page 6 MO Rules: Constructive overlap = bonding Destructive overlap = antibonding Number of MO’s = Number of p orbitals The MO energies are symmetrically distributed above and below the initial energies of the p orbitals. Half the MO’s are bonding, the other half antibonding.) So, now for 1,3-butadiene: There are 4 p orbitals to consider. The lowest energy MO will have the greatest number of favorable interactions (i.e. with all the p lobes interacting constructively). This is called 1. (Although 1,3-butadiene is not linear, it is easier and convenient to represent it as such). This MO places electron density on all four carbon atoms. There are 3 bonding interactions and this MO delocalizes the electrons over four nuclei. This explains why there is partial double bond character between C2 and C3. =