正在加载图片...
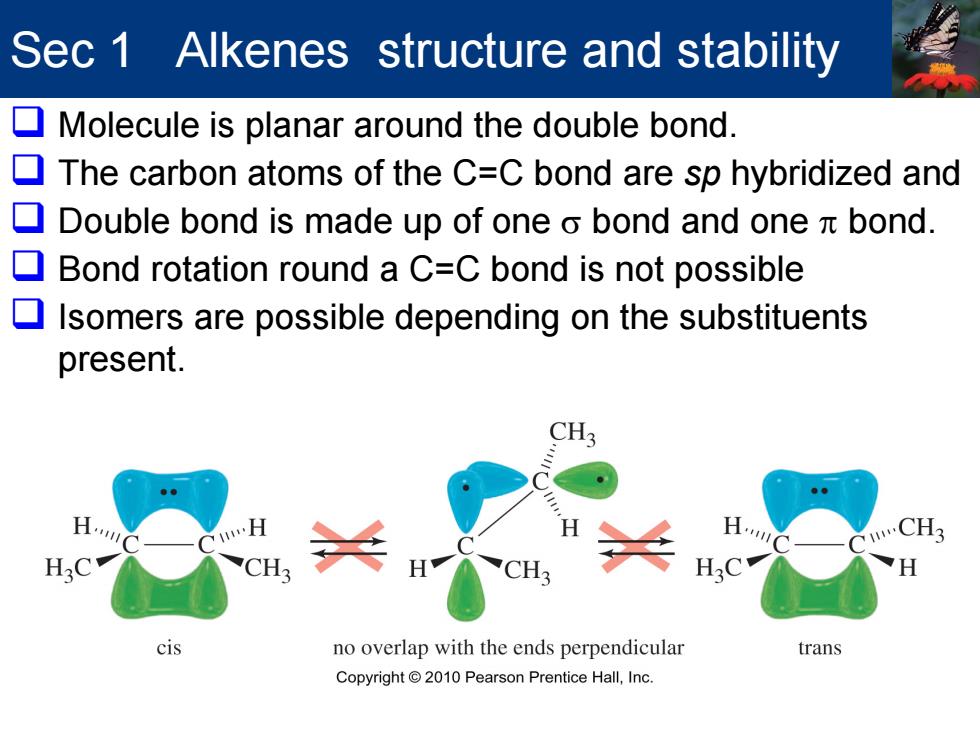
Sec 1 Alkenes structure and stability Molecule is planar around the double bond. 口 The carbon atoms of the C=C bond are sp hybridized and Double bond is made up of one o bond and one bond. Bond rotation round a C=C bond is not possible Isomers are possible depending on the substituents present. CH3 CH3 cis no overlap with the ends perpendicular trans Copyright 2010 Pearson Prentice Hall,Inc. Sec 1 Alkenes structure and stability Molecule is planar around the double bond. The carbon atoms of the C=C bond are sp hybridized and Double bond is made up of one σ bond and one π bond. Bond rotation round a C=C bond is not possible Isomers are possible depending on the substituents present