正在加载图片...
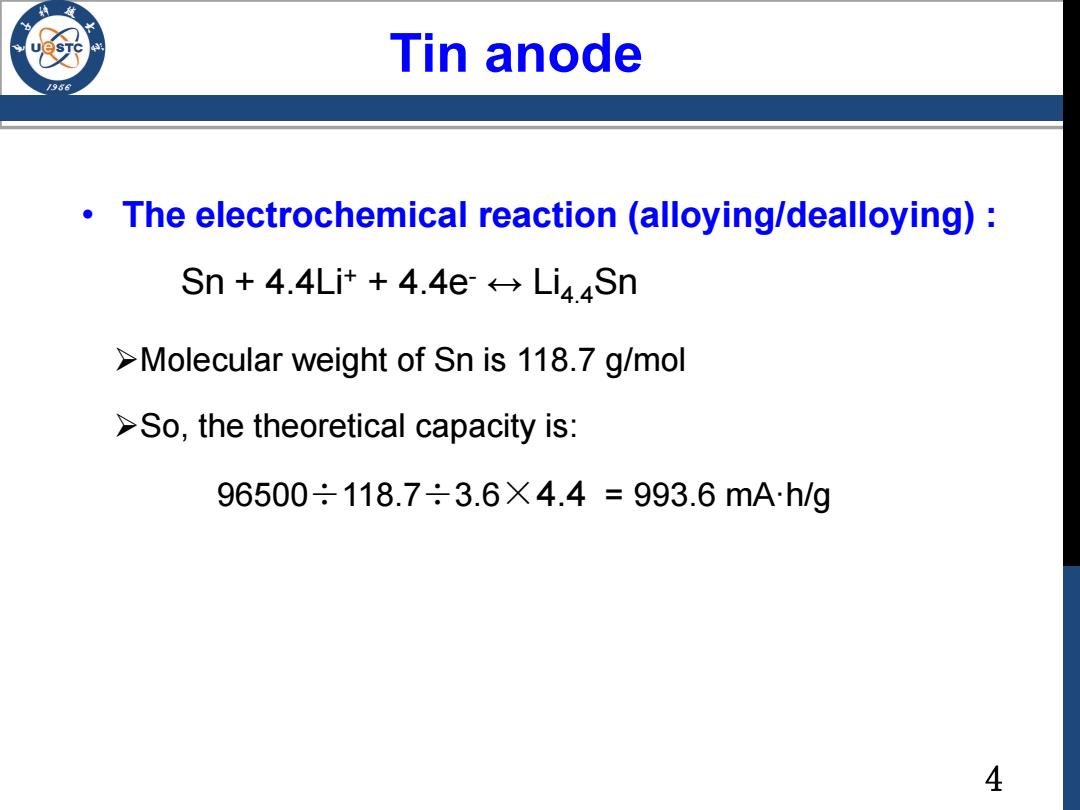
Tin anode /986 The electrochemical reaction (alloying/dealloying): Sn 4.4Li++4.4e Li4Sn >Molecular weight of Sn is 118.7 g/mol >So,the theoretical capacity is: 96500÷118.7÷3.6X4.4=993.6mAh/g 44 • The electrochemical reaction (alloying/dealloying) : Sn + 4.4Li+ + 4.4e- ↔ Li4.4Sn Molecular weight of Sn is 118.7 g/mol So, the theoretical capacity is: 96500÷118.7÷3.6×4.4 = 993.6 mA·h/g Tin anode