
Lecture 9 196 Anode material for LIBs: Tin Chen Junsong School of Materials and Energy 2020.04
Anode material for LIBs: Tin Chen Junsong School of Materials and Energy 2020.04 Lecture 9
Content /986 Introduction of Tin 。 Chemistry of Tin Molecular structure and Li insertion Synthesis and modification methods One literature example 2
2 Content • Introduction of Tin • Chemistry of Tin • Molecular structure and Li insertion • Synthesis and modification methods • One literature example
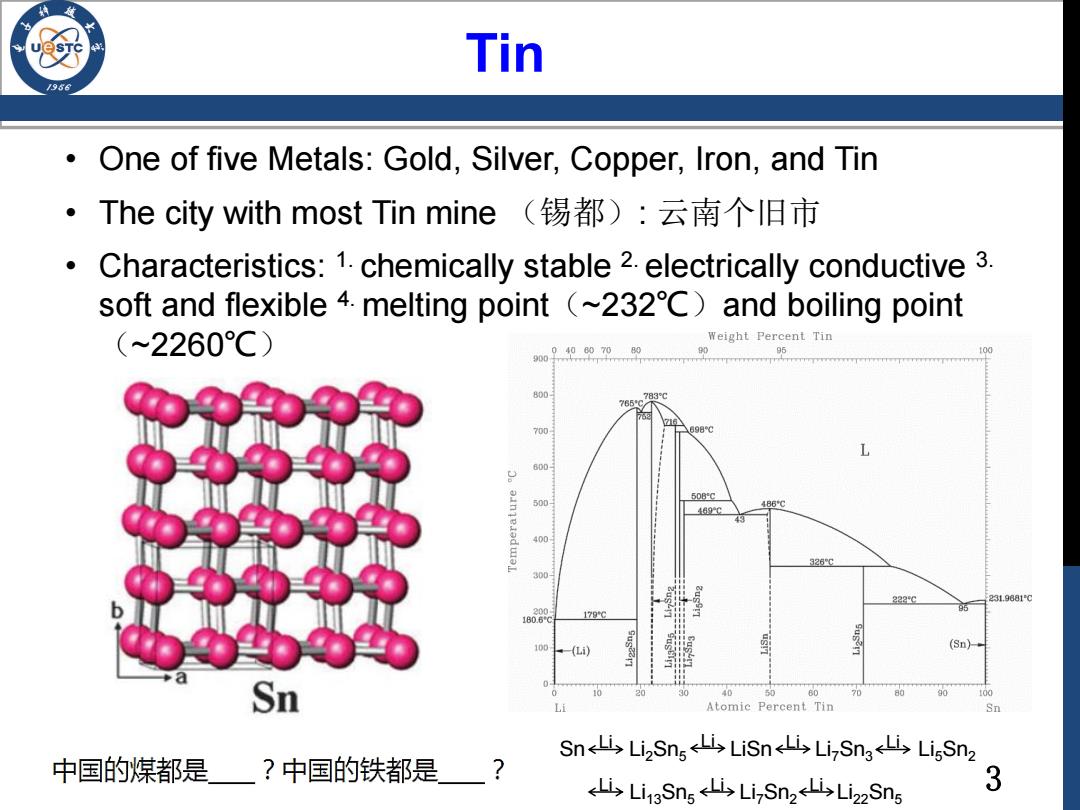
Tin One of five Metals:Gold,Silver,Copper,Iron,and Tin ·The city with most Tin mine(锡都):云南个旧市 Characteristics:1.chemically stable 2.electrically conductive 3. soft and flexible 4.melting point (~232C)and boiling point (~2260℃) Weight Percent Tin 9009406070 95 765 700 698C L 500 50BC 469c 486℃ 326 222C9 231.9081℃ 179G 10D (Sn)- Sn 40 60 100 Li Atomic Percent Tin Sn Sn<4Li2Sn,山LiSn<山Li,Sn3iLi5Snz 中国的煤都是?中国的铁都是 ? 山Li3Sne5 Lis Li,Sn2山Li2zSns 3
3 Tin Sn Li2Sn5 LiSn Li7Sn3 Li5Sn2 Li13Sn5 Li7Sn2 Li22Sn5 Li Li Li Li Li Li Li • One of five Metals: Gold, Silver, Copper, Iron, and Tin • The city with most Tin mine (锡都): 云南个旧市 • Characteristics: 1. chemically stable 2. electrically conductive 3. soft and flexible 4. melting point(~232℃)and boiling point (~2260℃)
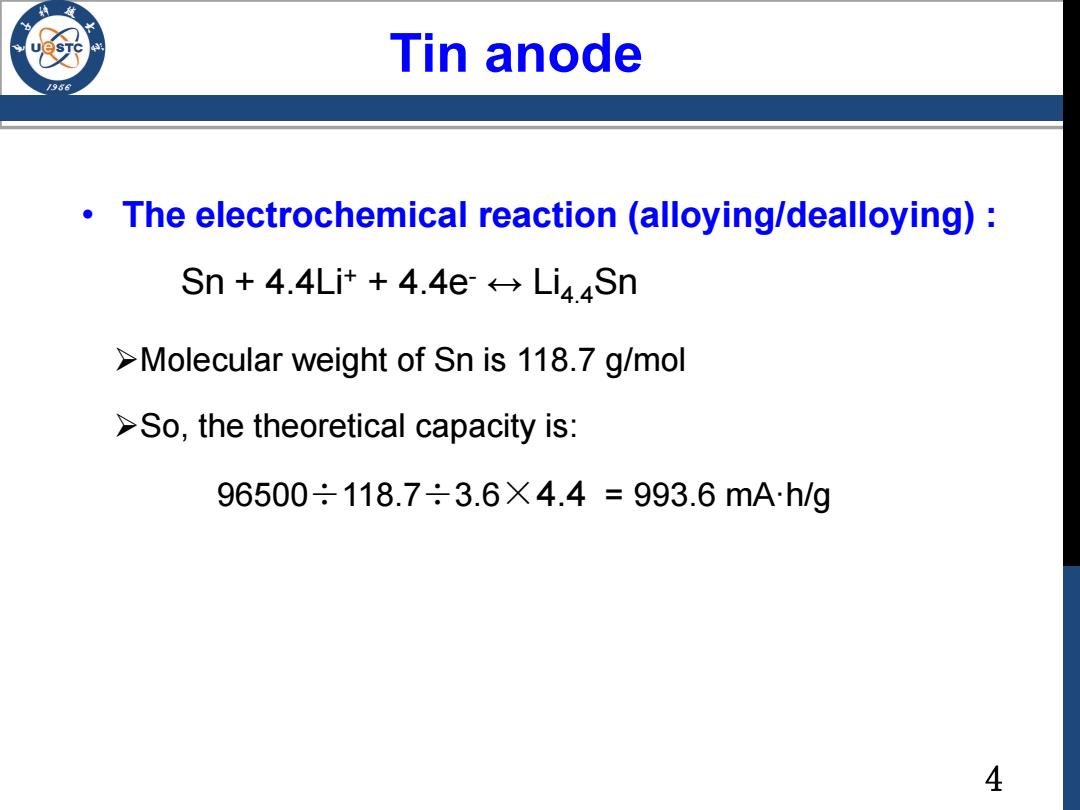
Tin anode /986 The electrochemical reaction (alloying/dealloying): Sn 4.4Li++4.4e Li4Sn >Molecular weight of Sn is 118.7 g/mol >So,the theoretical capacity is: 96500÷118.7÷3.6X4.4=993.6mAh/g 4
4 • The electrochemical reaction (alloying/dealloying) : Sn + 4.4Li+ + 4.4e- ↔ Li4.4Sn Molecular weight of Sn is 118.7 g/mol So, the theoretical capacity is: 96500÷118.7÷3.6×4.4 = 993.6 mA·h/g Tin anode
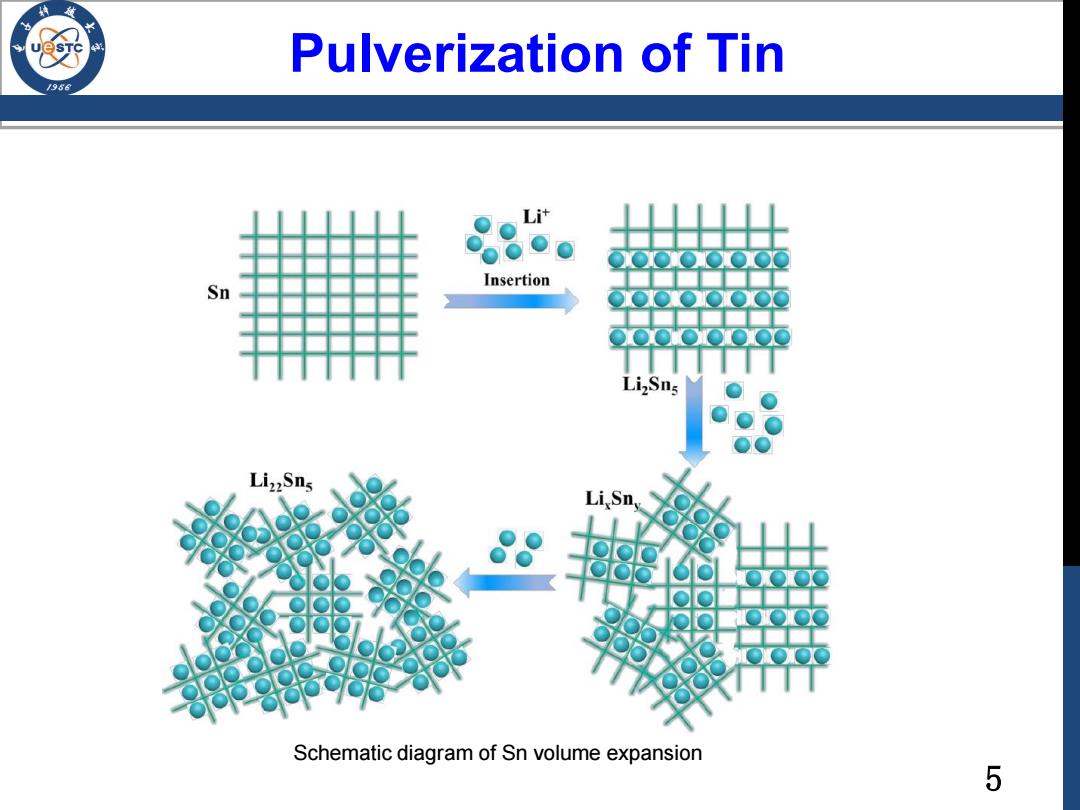
Pulverization of Tin /96 L Sn Insertion Li2Sns Liz2Sns Li Sn Schematic diagram of Sn volume expansion 5
5 Pulverization of Tin Schematic diagram of Sn volume expansion
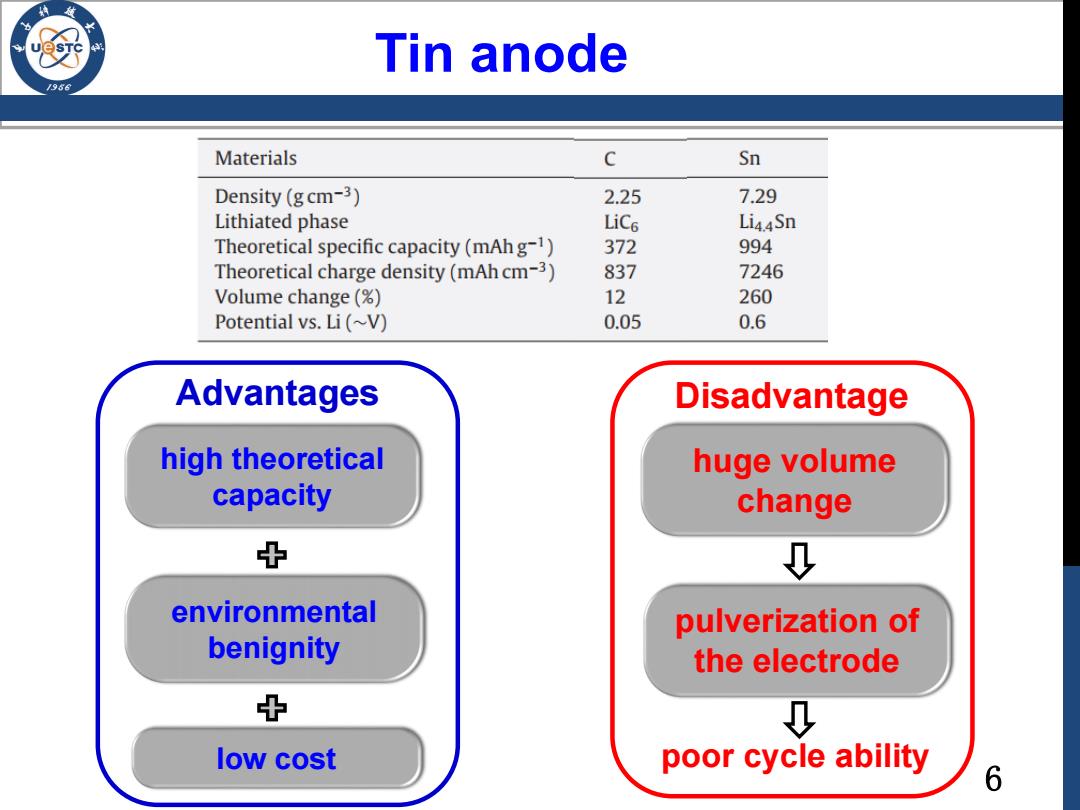
Tin anode 196 Materials c Sn Density(gcm-3) 2.25 7.29 Lithiated phase LiCe Li44Sn Theoretical specific capacity(mAh g-1) 372 994 Theoretical charge density(mAh cm-3) 837 7246 Volume change (% 12 260 Potential vs.Li(~V) 0.05 0.6 Advantages Disadvantage high theoretical huge volume capacity change 中 D environmental pulverization of benignity the electrode 中 low cost poor cycle ability 6
6 Tin anode high theoretical capacity environmental benignity Advantages Disadvantage huge volume change pulverization of the electrode low cost poor cycle ability

Tin anode 96 a 0.8 Li,Sns 入sn LiSn n/.n Li,Sna/Liz2Sns 0 20 40 60 Li content (at.%) apejins Nat.Mater..2013,12,1102 Potentiostatic dealloying (1 V versus Li/Li)results of Sn particle morphologies as a function of Li composition obtained by a single alloying/dealloying cycle in 1 M LiPF6 in ethylene carbonate/diethylcarbonate,1:1 v/v at room temperature.(a)Voltage versus Li content for alloying Li into ~2 um diameter Sn particles at a fixed current of-49.7 mA/gsn.The voltages define the composition of the particles.(b-g)The first row of SEM images corresponds to particle surfaces and the second row to focused ion-beam milled cross sections of the particles.(b,c)Li30Sn.70:the surface is roughened with void nodules,and the particle interior contains ~10 nm-diameter voids(c,inset,size of image,200 nm)that may have formed through a Kirkendall process.These voids are absent in virgin Sn particles.(d.e)Li4Sn52:collapsed bicontinuous morphology yielding a hollow core-shell-like7 structure.(f.g)Lio.Sn23:bicontinuous morphology with a ligament size of 50-100 nm.Scale bars,500 nm
7 Tin anode Potentiostatic dealloying (1 V versus Li+ /Li) results of Sn particle morphologies as a function of Li composition obtained by a single alloying/dealloying cycle in 1 M LiPF6 in ethylene carbonate/diethylcarbonate, 1:1 v/v at room temperature. (a) Voltage versus Li content for alloying Li into ∼2 μm diameter Sn particles at a fixed current of -49.7 mA/gSn. The voltages define the composition of the particles. (b-g) The first row of SEM images corresponds to particle surfaces and the second row to focused ion-beam milled cross sections of the particles. (b,c) Li0.30Sn0.70; the surface is roughened with void nodules, and the particle interior contains ∼10 nm-diameter voids (c, inset, size of image, 200 nm) that may have formed through a Kirkendall process. These voids are absent in virgin Sn particles. (d,e) Li0.48Sn0.52; collapsed bicontinuous morphology yielding a hollow core-shell-like structure. (f,g) Li0.77Sn0.23; bicontinuous morphology with a ligament size of 50-100 nm. Scale bars, 500 nm Nat. Mater. 2013, 12, 1102

Tin anode /98 ·Solution: >Nanosizing nanosphere nanocube nanowire nanotube .. >Coating Sn@C(good electronic conductivity,prevent aggregation, accommodate the strain of volume change)Sn@PPy .. >Alloying Sn-Cu Sn-Ni Sn-Sb Sn-Ni-Cu .. 8
8 Tin anode • Solution: Nanosizing nanosphere nanocube nanowire nanotube … Coating Sn@C(good electronic conductivity, prevent aggregation, accommodate the strain of volume change) Sn@PPy … Alloying Sn-Cu Sn-Ni Sn-Sb Sn-Ni-Cu …
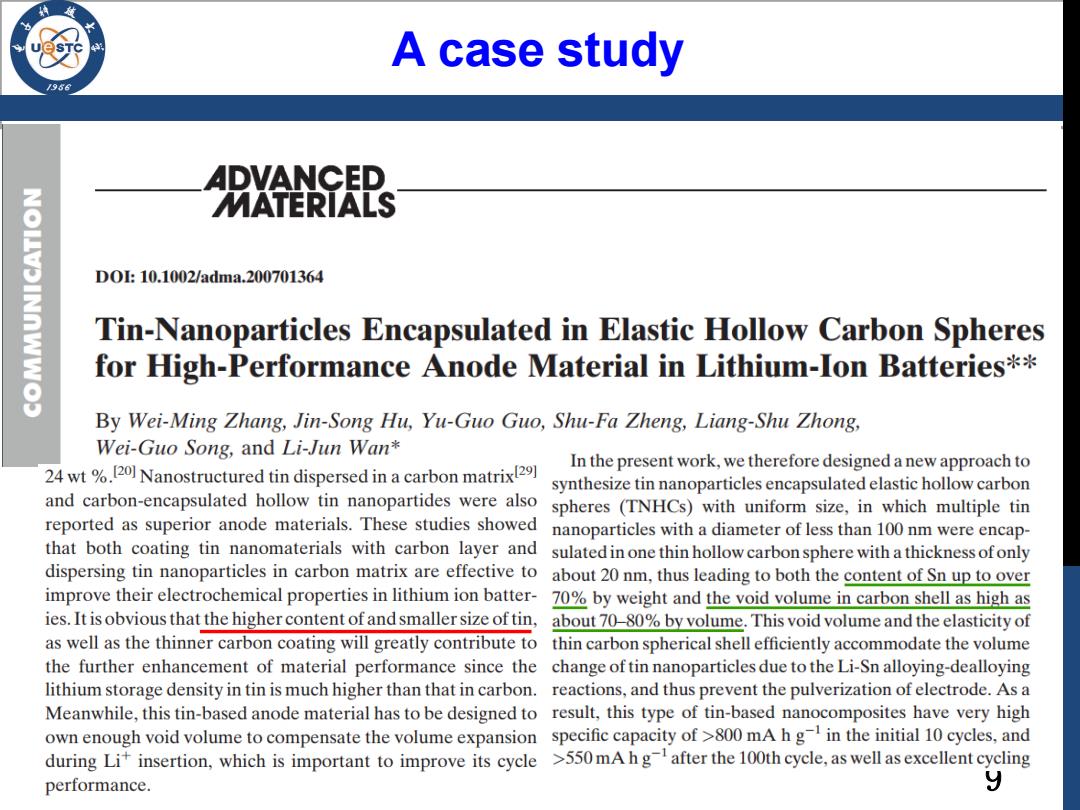
A case study 思 D0:10.1002/adma.200701364 Tin-Nanoparticles Encapsulated in Elastic Hollow Carbon Spheres for High-Performance Anode Material in Lithium-Ion Batteries** By Wei-Ming Zhang,Jin-Song Hu,Yu-Guo Guo,Shu-Fa Zheng,Liang-Shu Zhong, Wei-Guo Song,and Li-Jun Wan* 24wt%20 Nanostructured tin dispersed in a carbon matrix In the present work,we therefore designed a new approach to synthesize tin nanoparticles encapsulated elastic hollow carbon and carbon-encapsulated hollow tin nanopartides were also spheres (TNHCs)with uniform size,in which multiple tin reported as superior anode materials.These studies showed nanoparticles with a diameter of less than 100 nm were encap- that both coating tin nanomaterials with carbon layer and sulated in one thin hollow carbonsphere with a thickness ofonly dispersing tin nanoparticles in carbon matrix are effective to about 20 nm,thus leading to both the content of Sn up to over improve their electrochemical properties in lithium ion batter-70%by weight and the void volume in carbon shell as high as ies.It is obvious that the higher content of and smaller size of tin,about 70-80%by volume.This void volume and the elasticity of as well as the thinner carbon coating will greatly contribute to thin carbon spherical shell efficiently accommodate the volume the further enhancement of material performance since the change of tin nanoparticles due to the Li-Sn alloying-dealloying lithium storage density in tin is much higher than that in carbon.reactions,and thus prevent the pulverization of electrode.As a Meanwhile,this tin-based anode material has to be designed to result,this type of tin-based nanocomposites have very high own enough void volume to compensate the volume expansion specific capacity of>800 mA h g in the initial 10 cycles,and during Lit insertion,which is important to improve its cycle >550mA hgafter the 100th cycle,as well as excellent cycling performance. y
9 A case study

Synthesis 196 SnO2+C-Sn(liquid)+CO2(gas) 700℃ surface tension shrunk and formed tin nano-droplets 0 adhered to the carbon shell ater cooling tin nanoparticles encapsulated elastic hollow carbon spheres (TNHCs) 10
10 Synthesis 700℃ shrunk and formed tin nano-droplets adhered to the carbon shell after cooling tin nanoparticles encapsulated elastic hollow carbon spheres (TNHCs) surface tension