
Lecture 6 196 Anode material for LIBs: Graphite Chen Junsong School of Materials and Energy 2020.04
Anode material for LIBs: Graphite Chen Junsong School of Materials and Energy 2020.04 Lecture 6
Content /986 Overview of anode materials Introduction of graphite 。 Chemistry of graphite Molecular structure and Li insertion Synthesis and modification methods One literature example 2
2 Content • Overview of anode materials • Introduction of graphite • Chemistry of graphite • Molecular structure and Li insertion • Synthesis and modification methods • One literature example

Overview /986 。 General requirement React with Li+at a low potential vs.Li/Lit With a high Lit storage capacity Category:storage mechanism Insertion/deinsertion:graphite,TiO2,etc. Alloy/dealloy:Sn,Tin,etc. Replacement reaction:Fe2O3,Co3O4,etc. 3
3 Overview • General requirement React with Li+ at a low potential vs. Li/Li+ With a high Li+ storage capacity • Category: storage mechanism • Insertion/deinsertion: graphite, TiO2 , etc. • Alloy/dealloy: Sn, Tin, etc. • Replacement reaction: Fe2O3 , Co3O4 , etc
Graphite /98 Crystalline allotrope of carbon,most stable form Characteristics:1.thermally and chemically stable 2.thermally and electrically conductive 3.lubricating Origin:graphite mines in Heilongjiang,Shandong Preparation:purification of graphite mine: >1)chemical:NaOH reaction neutralize using HCI >2)physical:annealing at high T without oxygen https://b2b.hc360.com/viewPics/s https://b2b.hc360.com/viewPics/s http://www.99114.com/pi upplyself_pics/80332485791.html upplyself_pics/80332485791.html cture/598650.html 4
http://www.99114.com/pi cture/598650.html https://b2b.hc360.com/viewPics/s upplyself_pics/80332485791.html https://b2b.hc360.com/viewPics/s upplyself_pics/80332485791.html 4 Graphite • Crystalline allotrope of carbon, most stable form • Characteristics: 1. thermally and chemically stable 2. thermally and electrically conductive 3. lubricating • Origin:graphite mines in Heilongjiang, Shandong • Preparation:purification of graphite mine: 1) chemical:NaOH reaction → neutralize using HCl 2) physical:annealing at high T without oxygen
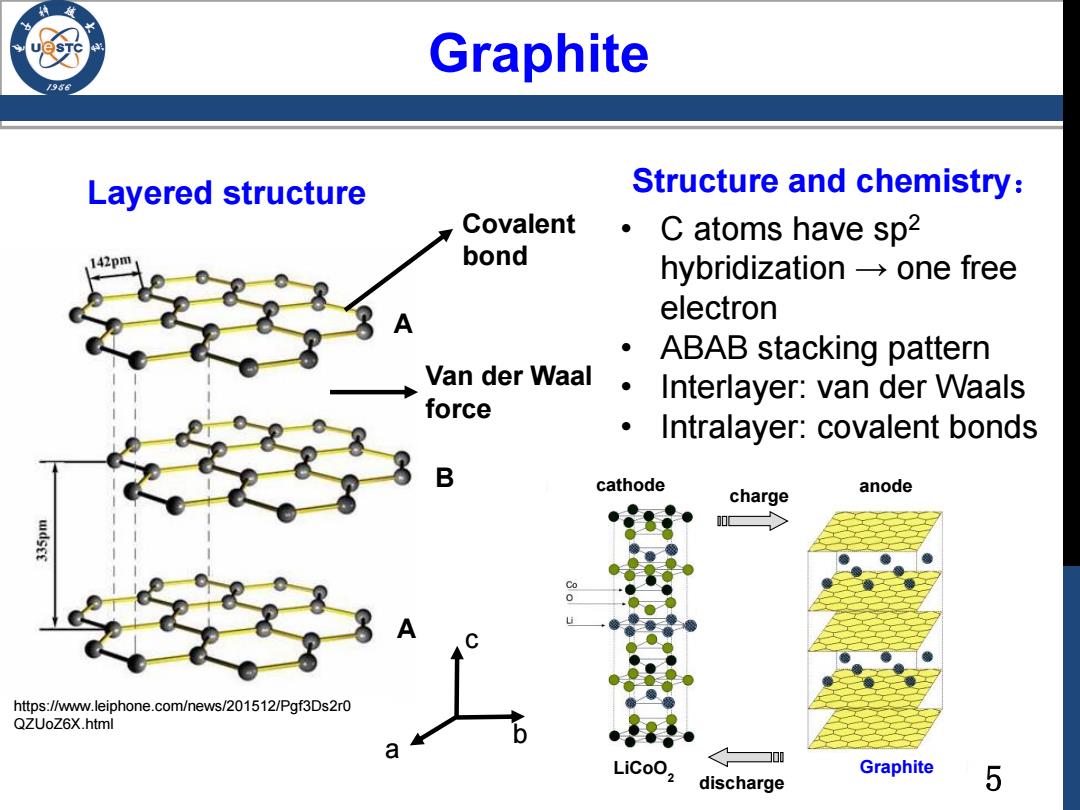
Graphite Layered structure Structure and chemistry: Covalent C atoms have sp2 42pm bond hybridization-one free electron ABAB stacking pattern Van der Waal 。 Interlayer:van der Waals force Intralayer:covalent bonds B cathode charge anode https://www.leiphone.com/news/201512/Pgf3Ds2r0 QZUoZ6X.html a LiCoO2 Graphite discharge 5
5 • C atoms have sp2 hybridization → one free electron • ABAB stacking pattern • Interlayer: van der Waals • Intralayer: covalent bonds A B A https://www.leiphone.com/news/201512/Pgf3Ds2r0 QZUoZ6X.html Layered structure Covalent bond Van der Waal force Structure and chemistry: charge discharge cathode LiCoO2 Graphite anode Graphite a b c
Graphite anode /98 The electrochemical reaction: C+1/6Lit+1/6e←→1/6LiC6 Reversible process (insertion/deinsertion) >Lit ions insert into(charge)and deinsert from (discharge) the interlayer space of graphite >Only the ions stored in the interlayer space can reversibly take part in the charge/discharge process 6
6 Graphite anode • The electrochemical reaction: C + 1/6Li+ + 1/6e- ↔ 1/6LiC6 • Reversible process (insertion/deinsertion) Li+ ions insert into (charge) and deinsert from (discharge) the interlayer space of graphite Only the ions stored in the interlayer space can reversibly take part in the charge/discharge process

Theoretical capacity of graphite /98 The electrochemical reaction: C+1/6Lit+1/6e←→1/6LiC6 >Molecular weight of C is 12 g/mol >So,the theoretical capacity is: 96500÷12÷3.6×1/6=372.3mAh/g 7
7 Theoretical capacity of graphite • The electrochemical reaction: C + 1/6Li+ + 1/6e- ↔ 1/6LiC6 Molecular weight of C is 12 g/mol So, the theoretical capacity is: 96500÷12÷3.6×1/6 = 372.3 mA·h/g

Properties of graphite 196 Advantages Disadvantage Low cost Working voltage too low + Electrochemically Poor rate stable performance ·Lit+6C+eLiC6 Solution: Rational structure design 06 x in LigCo 8
8 Properties of graphite Low cost Electrochemically stable Advantages Disadvantage Working voltage too low Poor rate performance Solution: Rational structure design
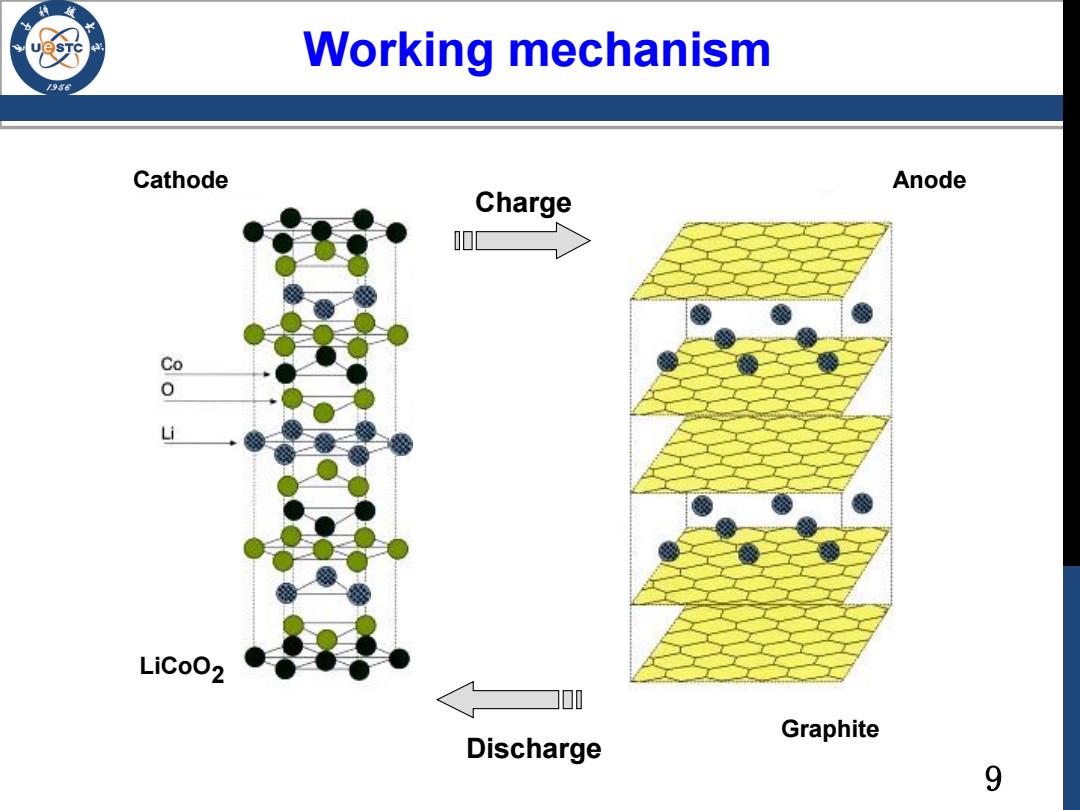
Working mechanism /986 Cathode Anode Charge LiCoO2 00 Graphite Discharge 9
9 Charge Discharge Cathode Anode Graphite LiCoO2 Working mechanism

A case study nature ARTICLES energy PUBLISHED:4 JULY 2016|ARTICLE NUMBER:16097 DOI:10.1038/NENERGY.2016.97 Magnetically aligned graphite electrodes for high-rate performance Li-ion batteries Juliette Billaud1,Florian Bouville2,Tommaso Magrini2,Claire Villevieille1*and Andre R.Studart2* As lithium-ion batteries become ubiquitous,the energy storage market is striving for better performance,longer lifetime and better safety of the devices.This race for performance is often focused on the search for new materials,whereas less effort has been dedicated to the electrode engineering.Enhancing the power density by increasing the amount of active material remains impractical since it impinges the transport of ions across the electrode during the charging and discharging processes.Here,we show that the electrochemical performance of a battery containing a thick (about 200 um),highly loaded (about 10 mg cm-2) graphite electrode can be remarkably enhanced by fabricating anodes with an out-of-plane aligned architecture using a low external magnetic field.The lower tortuosity resulting from such a simple and scalable magnetic alignment approach leads to a specific charge up to three times higher than that of non-architectured electrodes at a rate of 1C. 10
10 A case study