
例 Lecture 13 86 Solid-state Electrolyte in Li-ion Batteries Chen Junsong School of Materials and Energy 2020.04
Solid-state Electrolyte in Li-ion Batteries Chen Junsong School of Materials and Energy 2020.04 Lecture 13
Content 6 Why solid-state electrolyte(SSE)? ·Requirements of SSE History and Category of SSE Examples of SSE in each type 2
2 Content • Why solid-state electrolyte (SSE)? • Requirements of SSE • History and Category of SSE • Examples of SSE in each type
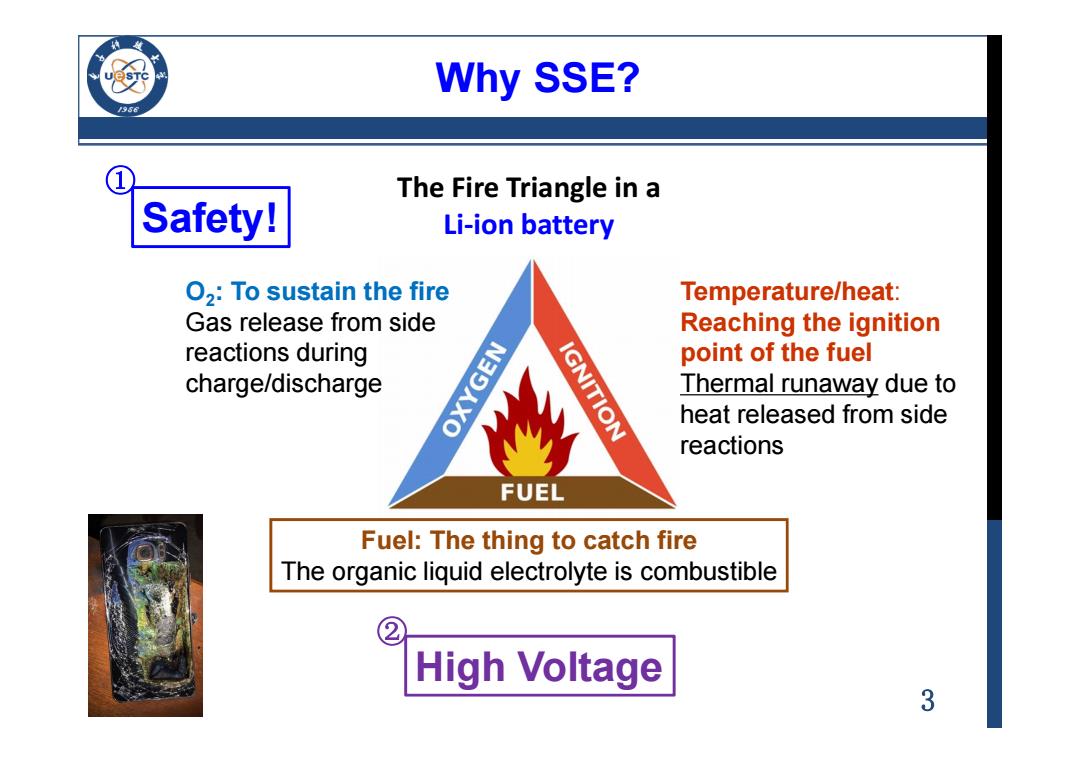
Why SSE? /956 ① The Fire Triangle in a Safety! Li-ion battery O2:To sustain the fire Temperature/heat: Gas release from side Reaching the ignition reactions during point of the fuel charge/discharge OXYGEN IGNITION Thermal runaway due to heat released from side reactions FUEL Fuel:The thing to catch fire The organic liquid electrolyte is combustible High Voltage 3
3 Why SSE? Safety! Fuel: The thing to catch fire The organic liquid electrolyte is combustible O2 : To sustain the fire Gas release from side reactions during charge/discharge Temperature/heat: Reaching the ignition point of the fuel Thermal runaway due to heat released from side reactions The Fire Triangle in a Li-ion battery High Voltage ① ②
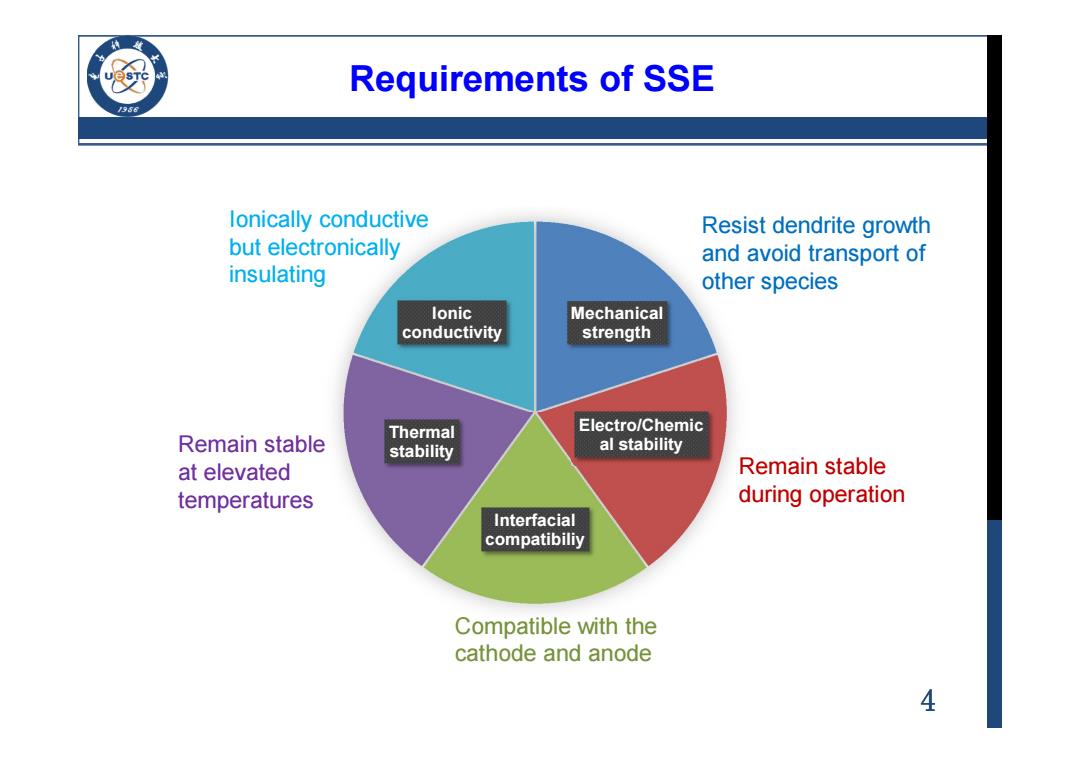
Requirements of SSE 956 lonically conductive Resist dendrite growth but electronically and avoid transport of insulating other species lonic Mechanical conductivity strength Remain stable Thermal Electro/Chemic stability al stability at elevated Remain stable temperatures during operation Interfacial compatibiliy Compatible with the cathode and anode 4
4 Requirements of SSE Mechanical strength Electro/Chemic al stability Interfacial compatibiliy Thermal stability Ionic conductivity Ionically conductive but electronically insulating Resist dendrite growth and avoid transport of other species Remain stable during operation Compatible with the cathode and anode Remain stable at elevated temperatures
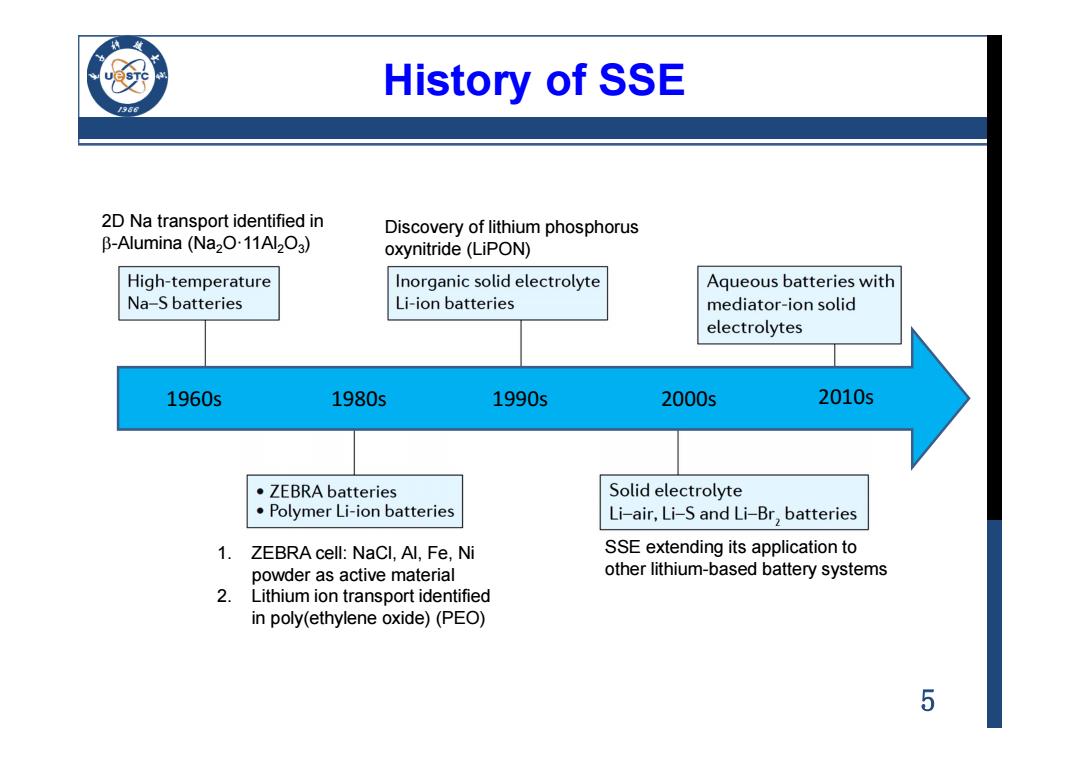
History of SSE 2D Na transport identified in Discovery of lithium phosphorus B-Alumina (Na20.11Al2O3) oxynitride(LiPON) High-temperature Inorganic solid electrolyte Aqueous batteries with Na-S batteries Li-ion batteries mediator-ion solid electrolytes 1960s 1980s 1990s 2000s 2010s ·ZEBRA batteries Solid electrolyte Polymer Li-ion batteries Li-air,Li-S and Li-Br,batteries 1.ZEBRA cell:NaCl,Al,Fe,Ni SSE extending its application to powder as active material other lithium-based battery systems 2.Lithium ion transport identified in poly(ethylene oxide)(PEO) 5
5 History of SSE 1960s 2D Na transport identified in b-Alumina (Na2O·11Al2O3 ) 1980s 1990s 2000s 2010s 1. ZEBRA cell: NaCl, Al, Fe, Ni powder as active material 2. Lithium ion transport identified in poly(ethylene oxide) (PEO) Discovery of lithium phosphorus oxynitride (LiPON) SSE extending its application to other lithium-based battery systems
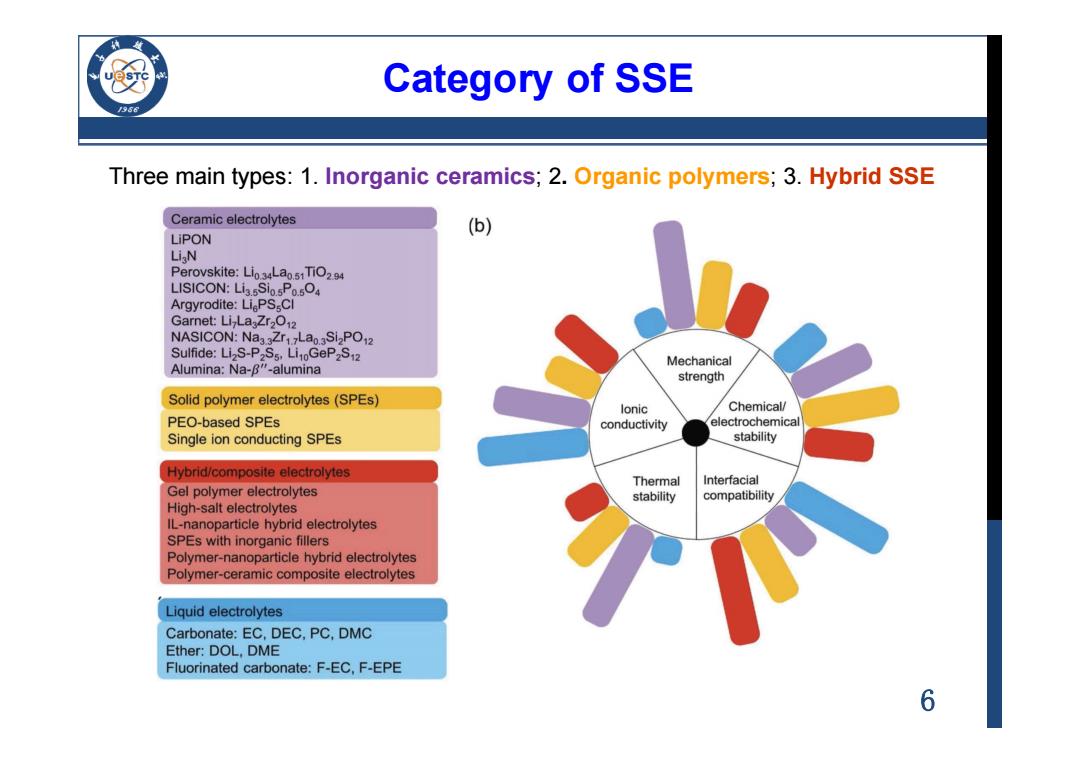
Category of SSE /956 Three main types:1.Inorganic ceramics;2.Organic polymers;3.Hybrid SSE Ceramic electrolytes (b) LiPON Li N Perovskite:Lio4Lao.51TiO24 LISICON:LissSiosPo.sO4 Argyrodite:LiPS CI Garnet:Li-LaZr2O12 NASICON:Na3.3Zr1.Lao.3Si2PO12 Sulfide:Li S-P2Ss.Li0GeP2S12 Mechanical Alumina:Na-B"-alumina strength Solid polymer electrolytes(SPEs) lonic Chemical/ PEO-based SPEs conductivity electrochemical Single ion conducting SPEs stability Hybrid/composite electrolytes Thermal Interfacial Gel polymer electrolytes stability compatibility High-salt electrolytes IL-nanoparticle hybrid electrolytes SPEs with inorganic fillers Polymer-nanoparticle hybrid electrolytes Polymer-ceramic composite electrolytes Liquid electrolytes Carbonate:EC,DEC,PC,DMC Ether:DOL,DME Fluorinated carbonate:F-EC,F-EPE 6
6 Category of SSE Three main types: 1. Inorganic ceramics; 2. Organic polymers; 3. Hybrid SSE
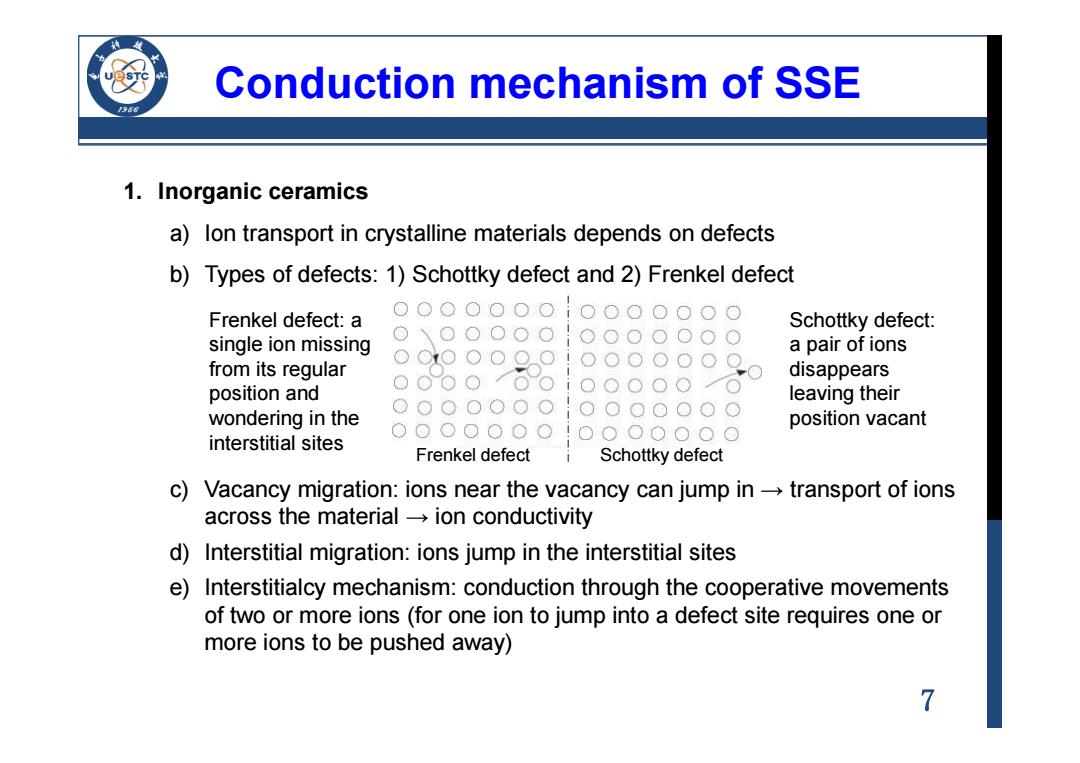
Conduction mechanism of SSE 1.Inorganic ceramics a)lon transport in crystalline materials depends on defects b)Types of defects:1)Schottky defect and 2)Frenkel defect Frenkel defect:a Schottky defect: single ion missing a pair of ions from its regular disappears position and leaving their wondering in the position vacant interstitial sites Frenkel defect Schottky defect c)Vacancy migration:ions near the vacancy can jump in-transport of ions across the materialion conductivity d)Interstitial migration:ions jump in the interstitial sites e)Interstitialcy mechanism:conduction through the cooperative movements of two or more ions (for one ion to jump into a defect site requires one or more ions to be pushed away) 7
7 Conduction mechanism of SSE 1. Inorganic ceramics a) Ion transport in crystalline materials depends on defects b) Types of defects: 1) Schottky defect and 2) Frenkel defect c) Vacancy migration: ions near the vacancy can jump in → transport of ions across the material → ion conductivity d) Interstitial migration: ions jump in the interstitial sites e) Interstitialcy mechanism: conduction through the cooperative movements of two or more ions (for one ion to jump into a defect site requires one or more ions to be pushed away) Frenkel defect Schottky defect Frenkel defect: a single ion missing from its regular position and wondering in the interstitial sites Schottky defect: a pair of ions disappears leaving their position vacant

Conduction mechanism of SSE 2.Organic polymers a)Definition:Solid ionic conductors formed by the dissolution of salts in suitable high molecular weight polymers(solid polymer electrolyte:SPE) b)First developed SPE:poly(ethylene oxide) c)Two conduction theories: Free Volume Theory:As temperature increases,the polymer expands,and creates local empty space,free volume,where the ionic carriers,solvated molecules,or polymer segments can move. The overall movability of the material is determined by the total amount of free volume present in the material. Dynamic Bond Percolation:lons will hop between well-defined ion sites along the disordered polymer,which is undergoing constant motion itself. Li+conduction in PEO:"Lit is coordinated by oxygen on PEO segmental chains.With the breaking and forming of Li-O bonds and continuous segmental rearrangement,long-range lithium transport can be realized"(10.1021/acs.chemrev.9b00268) 8
8 Conduction mechanism of SSE 2. Organic polymers a) Definition: Solid ionic conductors formed by the dissolution of salts in suitable high molecular weight polymers (solid polymer electrolyte: SPE) b) First developed SPE: poly(ethylene oxide) c) Two conduction theories: Free Volume Theory: As temperature increases, the polymer expands, and creates local empty space, free volume, where the ionic carriers, solvated molecules, or polymer segments can move. The overall movability of the material is determined by the total amount of free volume present in the material. Dynamic Bond Percolation: Ions will hop between well-defined ion sites along the disordered polymer, which is undergoing constant motion itself. • Li+ conduction in PEO: “Li+ is coordinated by oxygen on PEO segmental chains. With the breaking and forming of Li−O bonds and continuous segmental rearrangement, long-range lithium transport can be realized” (10.1021/acs.chemrev.9b00268)
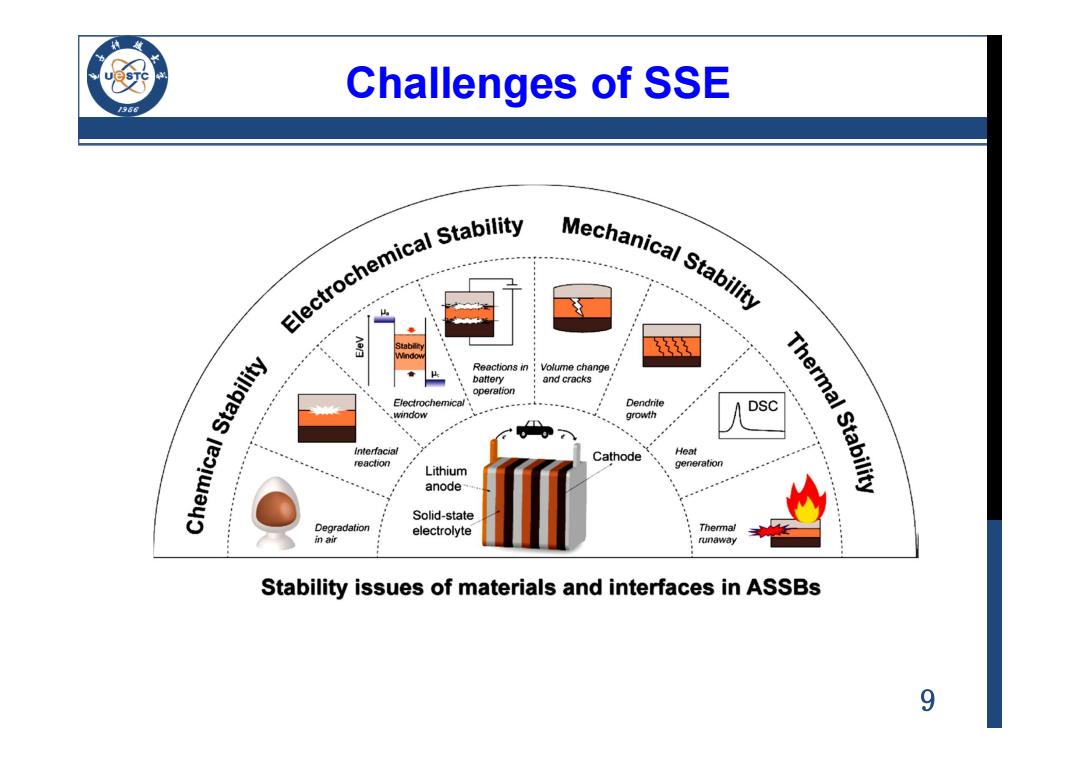
Challenges of SSE Electrochemical stability Mechanical Stabiy Volume change and cracks Chemical Stability operation Dendrite DSC .window growth interfacial Cathode Heat reaction generation Thermal Stability Lithium anode Solid-state Degradation electrolyte 加i runaway Stability issues of materials and interfaces in ASSBs 9
9 Challenges of SSE
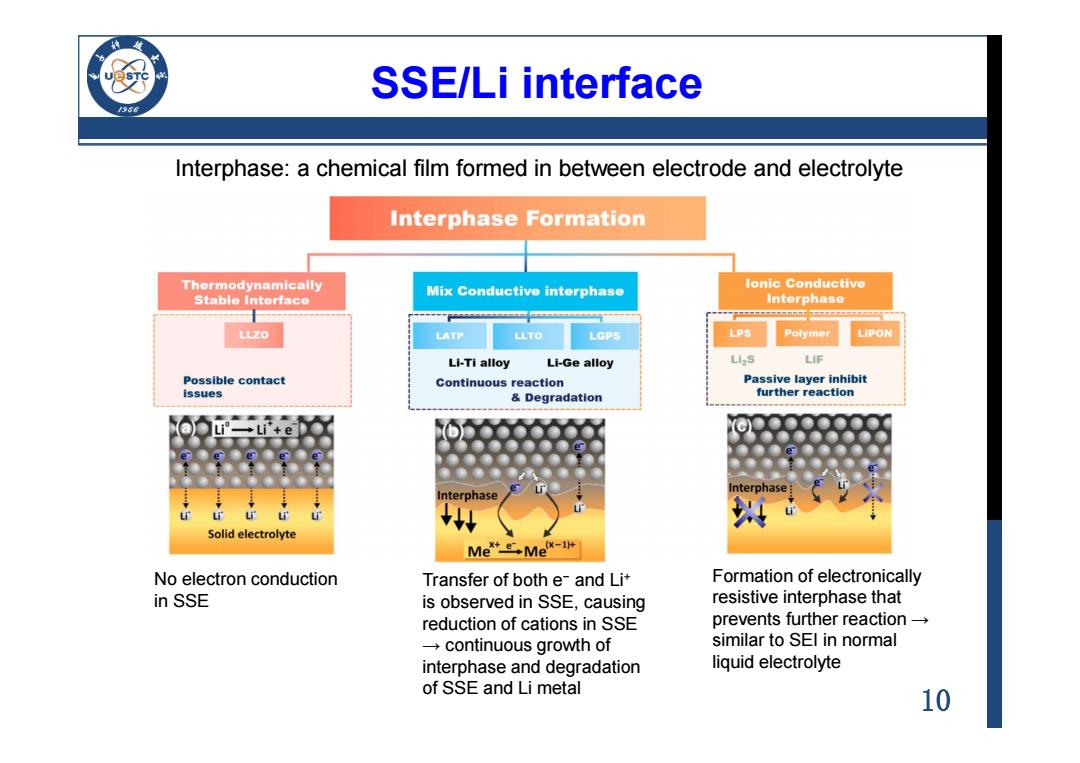
SSE/Li interface 96 Interphase:a chemical film formed in between electrode and electrolyte Interphase Formation Thermodynamically Mix Conductive interphase lonic Conductive Stable Interface Interphase LLZO LATP LLTO LGPS LPS Polymor Li-Ti alloy Li-Ge alloy LIS LIF Possible contact Continuous reaction Passive layer inhibit issues Degradation further reaction nterphas Interphase Solid electrolyte Me三,MeW-r No electron conduction Transfer of both e-and Lit Formation of electronically in SSE is observed in SSE,causing resistive interphase that reduction of cations in SSE prevents further reaction →continuous growth of similar to SEl in normal interphase and degradation liquid electrolyte of SSE and Li metal 10
10 SSE/Li interface Interphase: a chemical film formed in between electrode and electrolyte No electron conduction in SSE Transfer of both e− and Li+ is observed in SSE, causing reduction of cations in SSE → continuous growth of interphase and degradation of SSE and Li metal Formation of electronically resistive interphase that prevents further reaction → similar to SEI in normal liquid electrolyte