
Lecture 8 196 Anode Material for LIBs: Silicon Chen Junsong School of Materials and Energy 2020.04
Anode Material for LIBs: Silicon Chen Junsong School of Materials and Energy 2020.04 Lecture 8
Content /986 Introduction of silicon 0 Chemistry of silicon Reaction mechanisms of silicon anode 。Problems Synthesis and modification methods One literature example 2
2 Content • Introduction of silicon • Chemistry of silicon • Reaction mechanisms of silicon anode • Problems • Synthesis and modification methods • One literature example
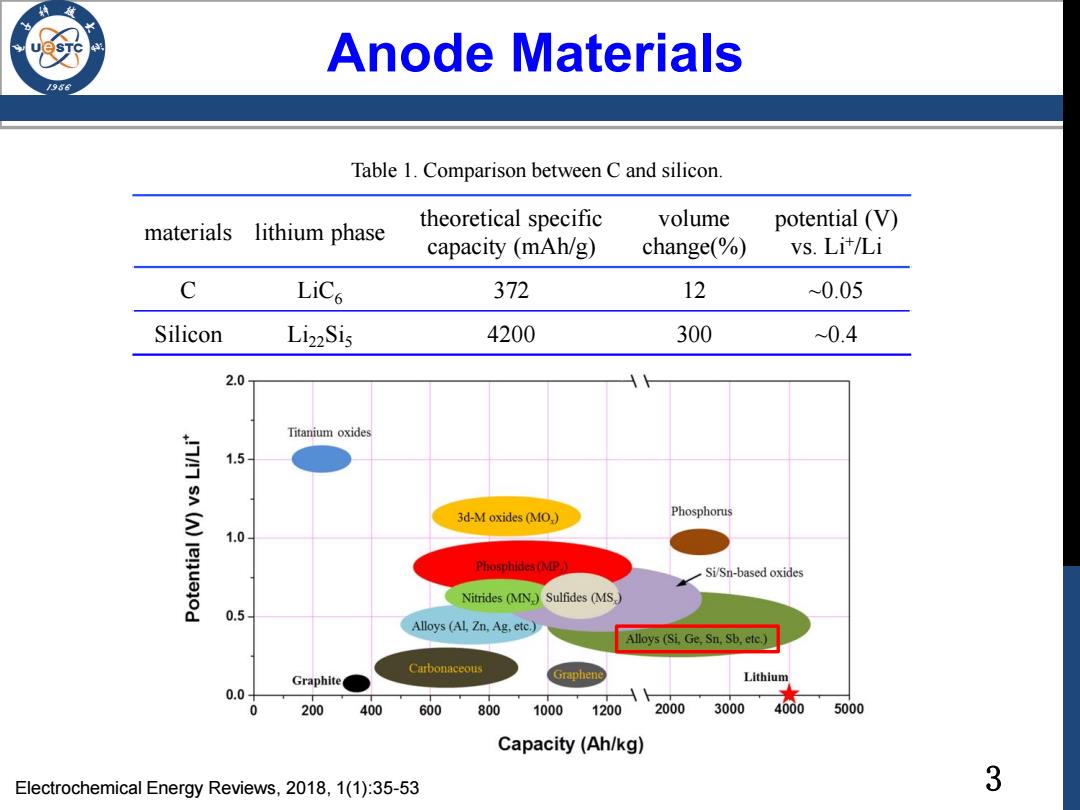
Anode Materials 936 Table 1.Comparison between C and silicon. materials lithium phase theoretical specific volume potential (V) capacity (mAh/g) change(%) vs.Lit/Li C LiCs 372 12 0.05 Silicon Li22Sis 4200 300 ~0.4 2.0 Titanium oxides 1.5 3d-M oxides(MO,) Phosphorus 1.0 Phosphides (MP. Si/Sn-based oxides Nitrides (MN,)Sulfides (MS,) 0.5 Alloys (AL,Zn.Ag.etc.) Alloys(Si.Ge,Sn,Sb,etc.) Carbonaceous Graphite Graphene Lithium 0.0 0 200 400 600 800 1000 1200 2000 3000 4000 5000 Capacity(Ah/kg) Electrochemical Energy Reviews,2018,1(1):35-53 3
3 Anode Materials materials lithium phase theoretical specific capacity (mAh/g) volume change(%) potential (V) vs. Li+ /Li C LiC6 372 12 ~0.05 Silicon Li22Si5 4200 300 ~0.4 Table 1. Comparison between C and silicon. Electrochemical Energy Reviews, 2018, 1(1):35-53

Silicon Origin:widely distributed in dusts,sands,clay in the forms of silicon dioxide(silica)or silicates Characteristics:1.amorphous and crystalline silicon,2.diamond cubic crystal structure,3semiconductor (E=1.12 eV) Preparation:conversion of silica(SiO2) >Carbothermal reduction→SiO,+2C→Si+2CO2 SiC+SiO2→3Si+2C0 >Aluminothermal reduction-3 SiO,+4 Al->3 Si+2 Al2O3 quartz,agate,agate
4 Silicon • Origin:widely distributed in dusts, sands, clay in the forms of silicon dioxide (silica) or silicates • Characteristics: 1. amorphous and crystalline silicon, 2. diamond cubic crystal structure, 3.semiconductor (Eg=1.12 eV) • Preparation:conversion of silica (SiO2 ) Carbothermal reduction → SiO2 + 2 C → Si + 2 CO2 SiC + SiO2 → 3 Si + 2 CO Aluminothermal reduction → 3 SiO2 + 4 Al → 3 Si + 2 Al2O3 quartz, agate, agate

Silicon Anode /98 The electrochemical reaction: >Si+4.4Li LiSi(fully lithiated state,each silicon atom can host 4.4 lithium atoms) Reversible process (lithiation/delithiation) >Lit ions insert into silicon and form Li-Si alloy(charge) Si→Li2.oSi→Li3sSi→Li44Si >Then deinsert from silicon and Li-Si dealloy (discharge) Li4,4Si→Li3sSi→Li2.oSi→Si 5
5 Silicon Anode • The electrochemical reaction: Si + 4.4Li+ ↔ Li4.4Si (fully lithiated state, each silicon atom can host 4.4 lithium atoms) • Reversible process (lithiation/delithiation) Li+ ions insert into silicon and form Li-Si alloy(charge) Si → Li2.0Si → Li3.5Si → Li4.4Si Then deinsert from silicon and Li-Si dealloy (discharge) Li4.4Si → Li3.5Si → Li2.0Si → Si

Advantages 95 High theoretical specific capacity Li22Sis-4200 mAh/g Low discharge potential plateau 0.4 V versus Lit/Li-high working voltage,leading to high energy density in a full LIB Environmentally friendly ·Low toxicity High abundance (the 2nd most abundant element in the earth's crust) 6
6 Advantages • High theoretical specific capacity Li22Si5—— 4200 mAh/g • Low discharge potential plateau ≈ 0.4 V versus Li+ /Li —— high working voltage, leading to high energy density in a full LIB • Environmentally friendly • Low toxicity • High abundance (the 2 nd most abundant element in the earth’s crust)
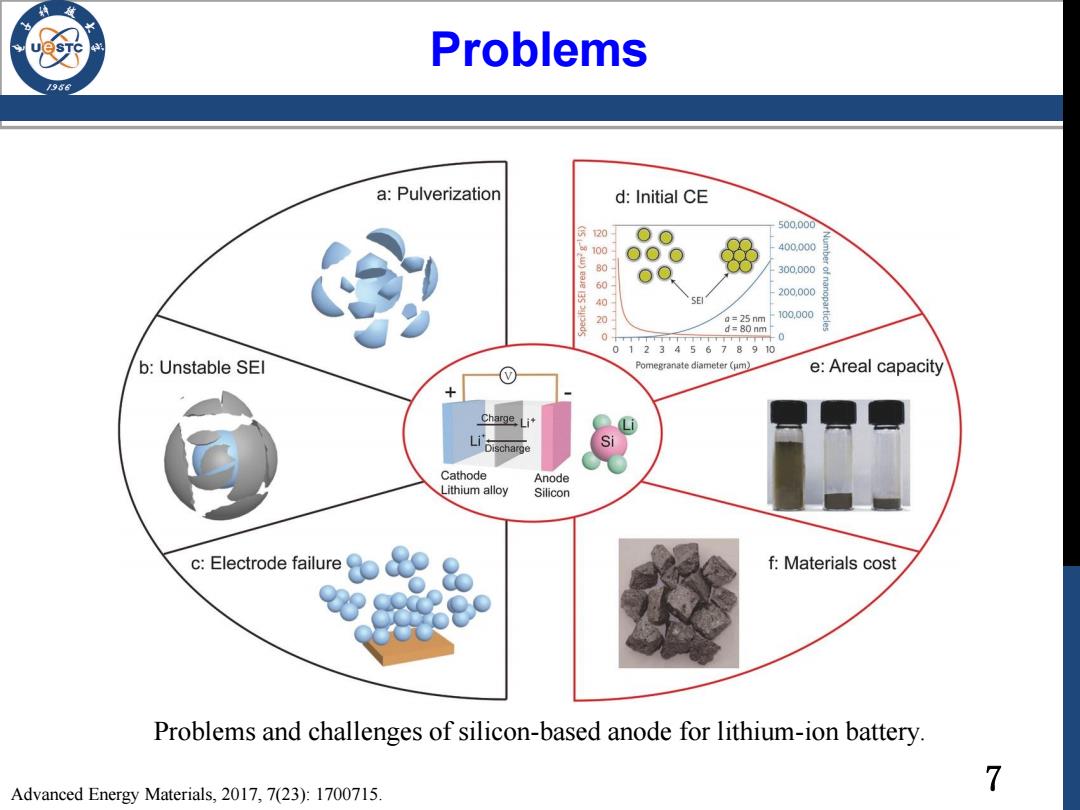
Problems /96 a:Pulverization d:Initial CE 500.000 20 oO w100 00O 400.000 80 o 300.000 60 200.000 40 E 20 a=25 nm 100.000 d=80 nm 0T斤 -0 012345678910 b:Unstable SEl Pomegranate diameter (um) e:Areal capacity Charge Li Discharge Si Cathode Anode Lithium alloy Silicon c:Electrode failure f:Materials cost Problems and challenges of silicon-based anode for lithium-ion battery. Advanced Energy Materials,2017,7(23):1700715. 7
7 Problems Problems and challenges of silicon-based anode for lithium-ion battery. Advanced Energy Materials, 2017, 7(23): 1700715
Problems Materials Pulverization Due to the large volume expansion,induces large stress,which can cause cracking and pulverization of Si,leading to loss of electrical contact and capacity fading. Unstable Solid Electrolyte Interphase The anode surface will be covered by an SEI film,the reductive decomposition of the organic electrolyte,it's electronically insulating but ionically conducting. Initial Coulombic Efficiency In the first cycle,active Li+is consumed due to the SEI film formation, resulting in irreversible capacity and extra cathode electrode consumption 8
8 Problems • Materials Pulverization Due to the large volume expansion, induces large stress, which can cause cracking and pulverization of Si, leading to loss of electrical contact and capacity fading. • Unstable Solid Electrolyte Interphase The anode surface will be covered by an SEI film, the reductive decomposition of the organic electrolyte, it’s electronically insulating but ionically conducting. • Initial Coulombic Efficiency In the first cycle, active Li+ is consumed due to the SEI film formation, resulting in irreversible capacity and extra cathode electrode consumption

Modification Methods 1986 Nano- structures Current Si-Based Collector 1.volume change 2.conductivity Composites 3.stable SEI Conductive Electrolyte Binder Additive 9
9 Modification Methods

Modification Methods 936 amorphous carbon self-healing Si coating microparticles Gen 9:Porous Si Gen 1:Si Nanowires Gen 5:Yolk-Shell Microparticles Gen 3:Hollow Nanospheres Gen 7:Self-Healing Gen 11:Conformal Polymer Coating Graphene Cages 2009 2011 2013 2015 2008 2010 2012 2014 2016 Timeline Gen 6:Conducting Gen 10:Prelithiation Hydrogel Framework Gen 2:Core-Shell Nanowires Gen 4:Double-Walled Gen 8:Pomegranate Nanotubes Hierarchical Structure Li,Si nanoparticles crystalline amorphous mechanical Si core Si shell constraining layer Roadmap of nanostructural designs for Si anodes. Accounts of Chemical Research,2017,50(12) 10
10 Modification Methods Accounts of Chemical Research, 2017, 50(12) Roadmap of nanostructural designs for Si anodes