
Lecture 12 /986 Safety of Li-ion Batteries Chen Junsong School of Materials and Energy 2020.04
Safety of Li-ion Batteries Chen Junsong School of Materials and Energy 2020.04 Lecture 12

Content /986 ·Why safety? Origins of safety concerns Thermal runaway process:3 stages Materials with improved battery safety Materials to solve problems in 3 stages 2
Content • Why safety? • Origins of safety concerns • Thermal runaway process: 3 stages • Materials with improved battery safety • Materials to solve problems in 3 stages 2

Why battery safety? 1956 Samsung Note 7 incidents PA F5 @层车 3
Why battery safety? Samsung Note 7 incidents 3
How to catch fire? TORCH The Fire Triangle O2:to sustain the fire OXYGEN IGNITION Temperature/heat:Reaching the ignition point of the fuel FUEL Flammable substance:the thing to catch fire Where do these things come from in a Li-ion battery? 4
How to catch fire? The Fire Triangle O2: to sustain the fire Temperature/heat: Reaching the ignition point of the fuel Flammable substance: the thing to catch fire Where do these things come from in a Li-ion battery? 4

Origins of safety concerns SCIENCE ADVANCES REVIEW MATERIALS SCIENCE Materials for lithium-ion battery safety Kai Liu',Yayuan Liu',Dingchang Lin',Allen Pei,Yi Cui2* Samsung Note 7 after the battery The Fire Triangle in a caught fire Li-ion battery O2:To sustain the fire Temperature/heat: Gas release from side Reaching the ignition reactions during charge/discharge OXYGEN point of the fuel IGNITION Thermal runaway due to heat released from side reactions FUEL Fuel:The thing to catch fire The organic liquid electrolyte is combustible 5
Origins of safety concerns Fuel: The thing to catch fire The organic liquid electrolyte is combustible O2: To sustain the fire Gas release from side reactions during charge/discharge Temperature/heat: Reaching the ignition point of the fuel Thermal runaway due to heat released from side reactions The Fire Triangle in a Li-ion battery Samsung Note 7 after the battery caught fire 5

The Thermal Runaway /986 Cathode- Anode Battery temperature increases Fires,explosions Separator and liquid electrolyte Stage 2:Heat accumulation and Stage 1:The onset of overheating gas release process Stage 3:Combustion and explosion Dendrite formation SEI decomposition (>90C) Oxygen Separator flaws Separator melt(>~130C) Overcharging Anode exposed(C.H gas release) Cell crush Liquid ↓ electrolyte combustion Temperature further increases ↓ Heat Fuel Big current Cathode decomposition,O2 release EC+DMC(1:1) (LiC002>~180C) Vapor pressure:4.8 kPa@R.T. Flash point::25℃±1C@1.013bar 6
The Thermal Runaway Dendrite formation Separator flaws Overcharging Cell crush Big current SEI decomposition (>90oC) Separator melt (>~130oC) Anode exposed (CxHy gas release) Temperature further increases Cathode decomposition, O2 release (LiCoO2 > ~180oC) EC+DMC(1:1) Vapor pressure: 4.8 kPa@R.T. Flash point: 25 oC±1 oC@1.013 bar 6
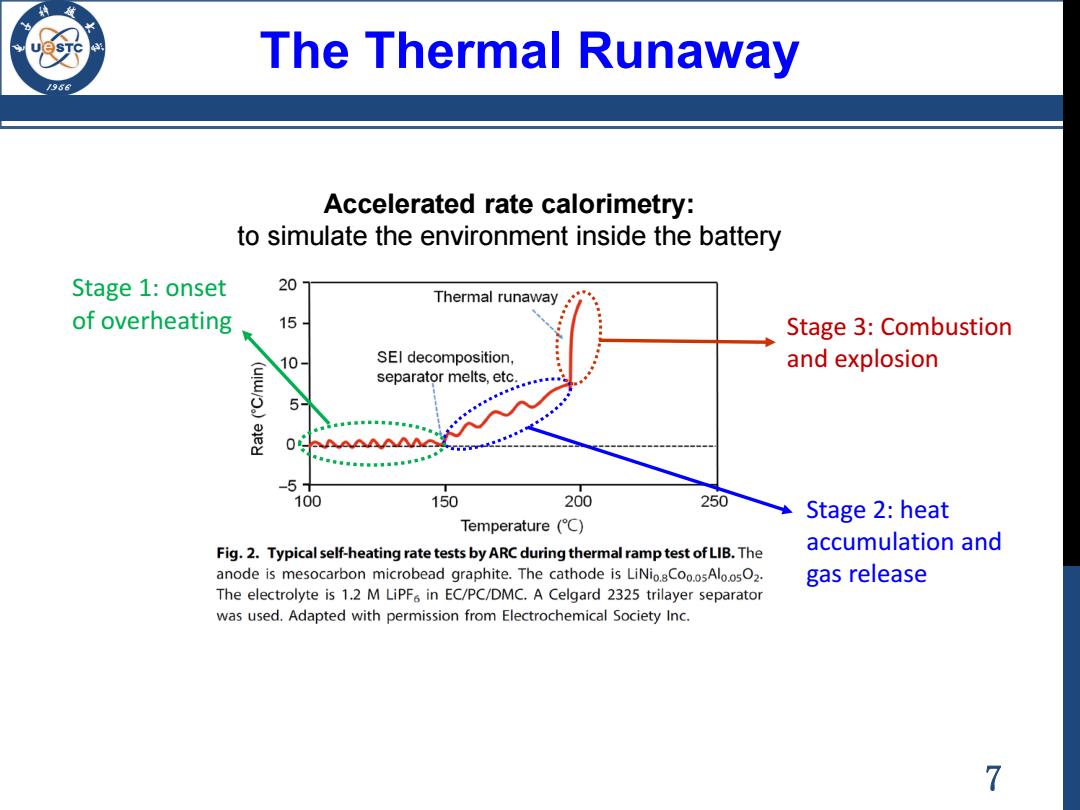
The Thermal Runaway 1956 Accelerated rate calorimetry: to simulate the environment inside the battery Stage 1:onset 20 Thermal runaway of overheating 15 Stage 3:Combustion 10 SEI decomposition, and explosion separator melts,etc. 5 0 MMG -5 100 150 200 250 Stage 2:heat Temperature (C) Fig.2.Typical self-heating rate tests by ARC during thermal ramp test of LIB.The accumulation and anode is mesocarbon microbead graphite.The cathode is LiNio.sCoo.osAlo.osO2. gas release The electrolyte is 1.2 M LiPF in EC/PC/DMC.A Celgard 2325 trilayer separator was used.Adapted with permission from Electrochemical Society Inc. 7
The Thermal Runaway Accelerated rate calorimetry: to simulate the environment inside the battery Stage 1: onset of overheating Stage 3: Combustion and explosion Stage 2: heat accumulation and gas release 7

Methods for safety 795 Lithium Stage 1:the onset of overheating Separator Overcharging Cell crush dendrite flaws 1.Reliable anode materials a)Use compounds like vinylene carbonate and maleimide to stabilize the SEI and eliminate the dendrite growth 2.Multi-functional liquid electrolyte and separator a)Fumed SiO,in electrolyte-Sheer thickening effect-viscosity t upon pressure-dissipating the impact A Velocity Velocity Damaged Battery cell 象 EC/DMC EL/DMC EC/DMC with LiPF with LiPFs with LiPFe Velocity Velocity Battery cell Silica clusters Normal EC/DMC Silica particles with LiPFs 8
Methods for safety Stage 1: the onset of overheating 1. Reliable anode materials a) Use compounds like vinylene carbonate and maleimide to stabilize the SEI and eliminate the dendrite growth 2. Multi-functional liquid electrolyte and separator a) Fumed SiO2 in electrolyte → Sheer thickening effect → viscosity ↑ upon pressure → dissipating the impact 8
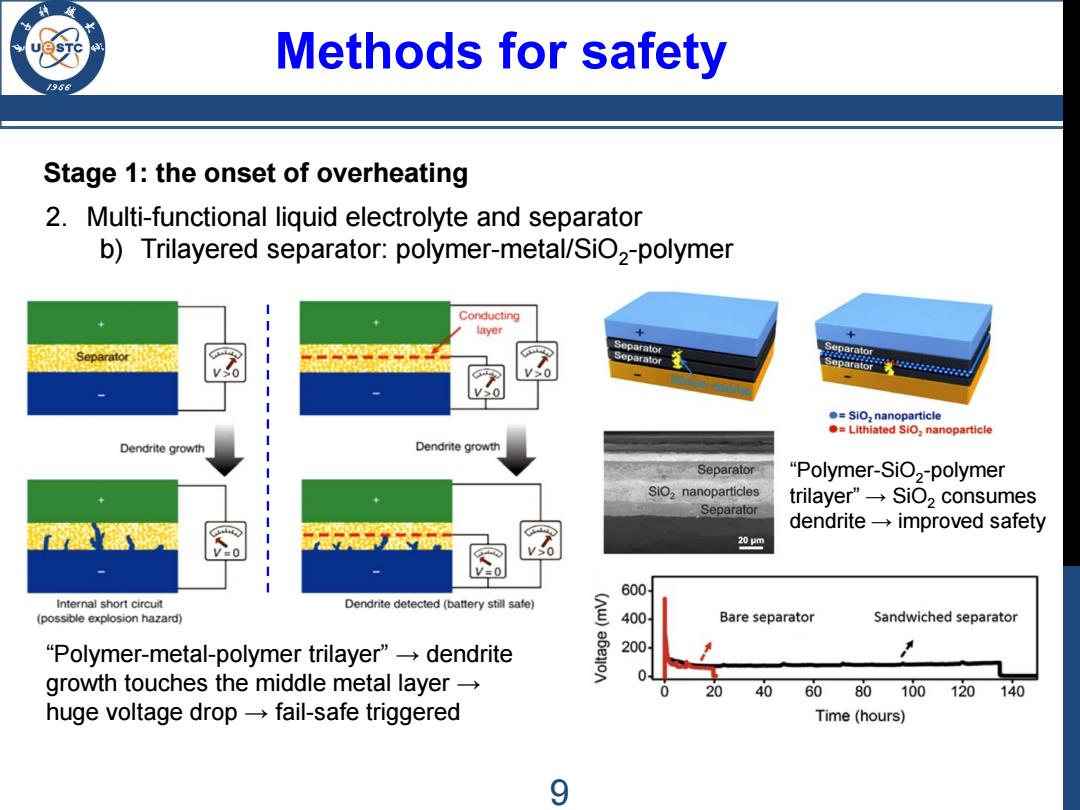
Methods for safety 9 Stage 1:the onset of overheating 2.Multi-functional liquid electrolyte and separator b)Trilayered separator:polymer-metal/SiO2-polymer Conducting layer parator 30 Separator 。=SiO,nanoparticle Lithiated SiO,nanoparticle Dendrite growth Dendrite growth Separator "Polymer-SiO,-polymer SiO,nanoparticles trilayer'”→SiO2 consumes Separator dendrite-improved safety 20m 600 Internal short circuit Dendrite detected (battery still safe) (possible explosion hazard) 400 Bare separator Sandwiched separator "Polymer-metal-polymer trilayer"dendrite 200 growth touches the middle metal layer 20 40 60 80100120 140 huge voltage drop-fail-safe triggered Time(hours) 9
Methods for safety Stage 1: the onset of overheating 2. Multi-functional liquid electrolyte and separator b) Trilayered separator: polymer-metal/SiO2-polymer “Polymer-metal-polymer trilayer” → dendrite growth touches the middle metal layer → huge voltage drop → fail-safe triggered “Polymer-SiO2-polymer trilayer” → SiO2 consumes dendrite → improved safety 9

Methods for safety Stage 1:the onset of overheating 3.( Overcharge protection(charging beyond designed voltage) a)Redox shuttle additives (reversible) Overcharged cathode e Anode Cathode e Diffusion 3.2V 3.5-3.7V 3.8V ◆A A Reduction Oxidation◆e A+ A+ (B12FxH12-x)2- Diffusion 4.3V 4.4V 4.5V >"oxidation-diffusion-reduction-diffusion" Different types of redox shuttle additives and Redox potential of additives should be their corresponding potential 0.3~0.4 V above the potential of the cathode > Tailoring the molecular structure-tune the potential 10
Methods for safety Stage 1: the onset of overheating 3. Overcharge protection (charging beyond designed voltage) a) Redox shuttle additives (reversible) “oxidation-diffusion-reduction-diffusion” Redox potential of additives should be 0.3~0.4 V above the potential of the cathode Overcharged cathode Different types of redox shuttle additives and their corresponding potential Tailoring the molecular structure → tune the potential 10