
Lecture 3 196 Lithium-ion Batteries Chen Junsong School of Materials and Energy 2020.04
Lithium-ion Batteries Chen Junsong School of Materials and Energy 2020.04 Lecture 3
Content /986 Lithium-metal batteries ·Safety problem Lithium-ion batteries Components,working mechanism ● Cathode materials:LiFePO4,Li-Mn-O 2
2 Content • Lithium-metal batteries • Safety problem • Lithium-ion batteries • Components, working mechanism • Cathode materials: LiFePO4 , Li-Mn-O

2019 Nobel Prize Chemistry THE NOBEL PRIZE Goodenough:discovery of LiCoO,and IN CHEMISTRY 2019 LiFePO,which become the cathode materials in the commercial LIBs now. Whittingham:discovery of layered TiS2 to reversibly intercalate lithium ions,and the concept of rechargeable Li battery. Yoshino:built the first rechargeable LIB John B. M.Stanley Akira by using LiCoO2 cathode and a graphite Goodenough Whittingham Yoshino anode. "for the development of lithium-ion batteries" THE ROYAL SWEDISH ACADEMY OF SCIENCES 3
3 2019 Nobel Prize Chemistry Goodenough: discovery of LiCoO2 and LiFePO4 , which become the cathode materials in the commercial LIBs now. Whittingham: discovery of layered TiS2 to reversibly intercalate lithium ions, and the concept of rechargeable Li battery. Yoshino: built the first rechargeable LIB by using LiCoO2 cathode and a graphite anode

196 4
4
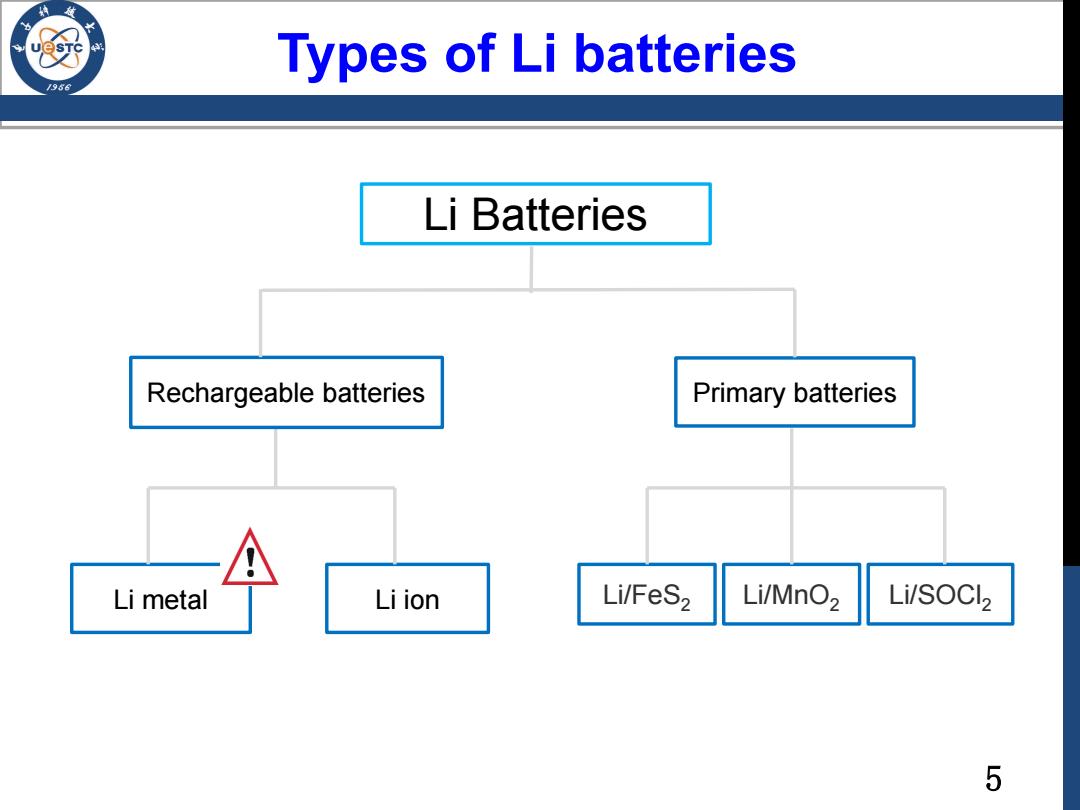
Types of Li batteries 196 Li Batteries Rechargeable batteries Primary batteries Li metal Liion Li/FeS2 Li/MnO2 Li/SOCl2 5
5 Li Batteries Li/FeS2 Li/MnO2 Li/SOCl Li metal Li ion 2 Types of Li batteries Rechargeable batteries Primary batteries

例 196 Li metal batteries 6
6 Li metal batteries

Advantage of Li /986 -Atomic weight: 18 H 6.94 g/mol He Heliom 16016- 13 14 15 16 17 403 Lightest alkali metal 9 10 Li (0.54g1/cm3) B C N 0 Ne u Cartcn Ncen 64941 012 1011 12511 14657 15 1R日 20.10 11 2 Silvery,metallic solid 14 15 16 18 Na Si Ar Theoretical capacity: Ah地m sfcca Salfar 24305 2692 3974 35453 3游94B 19 20 22 23 3.86 Ah/g 31 32 33 34 35 36 Ca Sc Ga Ge AS Se Br Kr Fetastiam Ttaniom ,线边n国国 Arserk Seknian 4007日 44956 50 72631 74922 78971 79904 37 38 39 40 41 E。=-3.04VsHE 49 50 51 52 53 Rb Sr Zr n Rab nt达ntra Rhodiam Cadnitm Sb Te Xe ttfmom 900 25% 9907 101.67 1028% 10642 1078e 112.410 1171 1217 1276 12690 1224 55 57-71 72 73 74 75 76 77 78 79 80 92 83 84 85 86 Cs Ba Hf W Re r Pt Au Hg c信u用 Oimlem risies fna间 M元 盟 Bi At Rn 129 13732 178.49 18u20 190.23 192217 15 1696 200592 2043☒ 2072 2e980 289 2099 2218 87 89-103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Ra Rf Db Bh Hs Mt Ds Rg Cn Uut Lv Uus Uuo tt与线m htr 2220 226025 261 26 B9 57 58 60 61 62 63 65 66 67 68 69 70 La Ce Pr Nd Pm Sm Eu Gd D Ho Er Tm Yb Lu a司fHn Terbiam Holnim Tbein T月a3un 和图等而 1905 140.116 140.90 1442日 144913 15036 151964 15725 1925 160 164930 16129 16934 1725g 1749%67 89 93 95 96 98 100 101 102 103 AC. Th a Np Am Cm Bk Cf Es Fm Md No Tortn a忙aem nengio Crin Beriellum Prlum 4otl柱与 2028 26 229 244064 243661 24070 2070 281 2511 lkal Metal Akalat山 Batie Metal emimeta国 7
7 - Atomic weight: 6.94 g/mol - Lightest alkali metal (0.54 g/cm3 ) - Silvery, metallic solid - Theoretical capacity: 3.86 Ah/g - Eo = -3.04 VSHE Advantage of Li
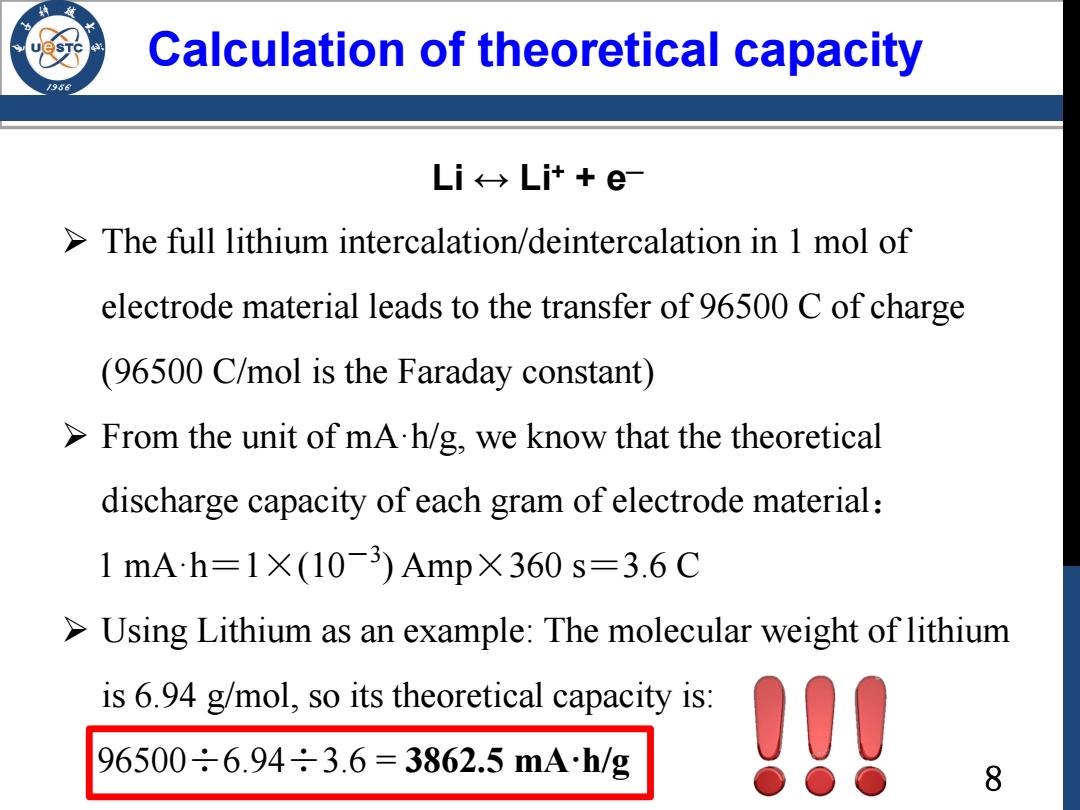
Calculation of theoretical capacity Li←→Lit+e- The full lithium intercalation/deintercalation in 1 mol of electrode material leads to the transfer of 96500 C of charge (96500 C/mol is the Faraday constant) >From the unit of mA.h/g,we know that the theoretical discharge capacity of each gram of electrode material: 1mAh=1×(10-3)Amp×360s=3.6C >Using Lithium as an example:The molecular weight of lithium is 6.94 g/mol,so its theoretical capacity is: 96500÷6.94÷3.6=3862.5m4h/g 09 8
8 Calculation of theoretical capacity The full lithium intercalation/deintercalation in 1 mol of electrode material leads to the transfer of 96500 C of charge (96500 C/mol is the Faraday constant) From the unit of mA·h/g, we know that the theoretical discharge capacity of each gram of electrode material: 1 mA·h=1×(10-3 ) Amp×360 s=3.6 C Using Lithium as an example: The molecular weight of lithium is 6.94 g/mol, so its theoretical capacity is: 96500÷6.94÷3.6 = 3862.5 mA·h/g Li ↔ Li+ + e─

Li metal batteries /96 Polymeric Li host Cathode Anode Separator allowing reversible intake/release of Lit What happens at the surface of Li metal?? (next slide...) Li intercalation Li metal compounds https://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ ImageService/Articleimage/2014/EE/c3ee40795k/c3ee40795k-f1_hi-res.gif 9
9 Li metal batteries https://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ ImageService/Articleimage/2014/EE/c3ee40795k/c3ee40795k-f1_hi-res.gif Polymeric Li host Separator allowing reversible intake/release of Li+ What happens at the surface of Li metal?? (next slide…)

Formation of Li dendrite 196 stripping Li 户 Lit e Plating Lithium deposition Lithium dissolution Cycling L Li计Li计L计 SEI layer Copper https://www.extremetech.com/wp-content/uploads/2014/07/1-9.jpg Increase in local Short circuit current and T 10
10 Formation of Li dendrite Li ⇌ Li+ + e─ https://www.extremetech.com/wp-content/uploads/2014/07/1-9.jpg stripping Plating Short circuit Increase in local current and T