
196 Anode material for LIB: TiO2 Chen Junsong School of Materials and Energy 2020.04
Anode material for LIB: TiO2 Chen Junsong School of Materials and Energy 2020.04
Content /986 ·Introduction of TiO2 。 Chemistry of TiO2 Molecular structure and Li insertion Synthesis and modification methods Several literature examples 2
2 Content • Introduction of TiO2 • Chemistry of TiO2 • Molecular structure and Li insertion • Synthesis and modification methods • Several literature examples
Titanium dioxide(TiO2) Low cost,environment friendly,biocompatibility,etc. Polymorphs:anatase,rutile,TiO(B),brookite ·Applications of TiO2 Solar Cell Photocatalysis Photocatalysis process @9 @ @ ⊙ Organic toxin Neuraizedidestroyed toin idation reactonts These reactonts decompose to through oidotion. 3
3 Titanium dioxide (TiO2 ) • Low cost, environment friendly, biocompatibility, etc. • Polymorphs: anatase, rutile, TiO2 (B), brookite • Applications of TiO2 Solar Cell Photocatalysis
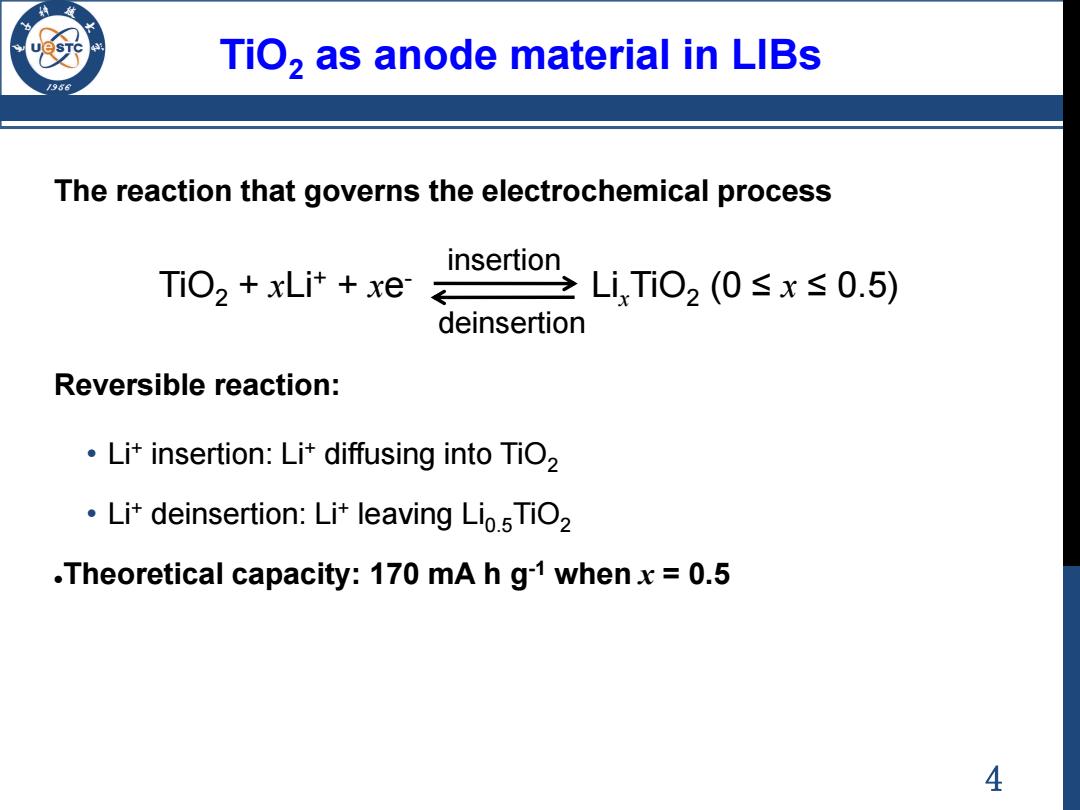
TiO,as anode material in LIBs 795 The reaction that governs the electrochemical process insertion TiO2 +xLit xe LiTi02(0≤x≤0.5) deinsertion Reversible reaction: Li+insertion:Lit diffusing into TiO2 Li+deinsertion:Lit leaving Lio.5TiO2 .Theoretical capacity:170 mA h g-1 whenx=0.5 4
4 TiO2 as anode material in LIBs The reaction that governs the electrochemical process TiO2 + xLi+ + xe - LixTiO2 (0 ≤ x ≤ 0.5) Reversible reaction: • Li+ insertion: Li+ diffusing into TiO2 • Li+ deinsertion: Li+ leaving Li0.5TiO2 Theoretical capacity: 170 mA h g-1 when x = 0.5 insertion deinsertion
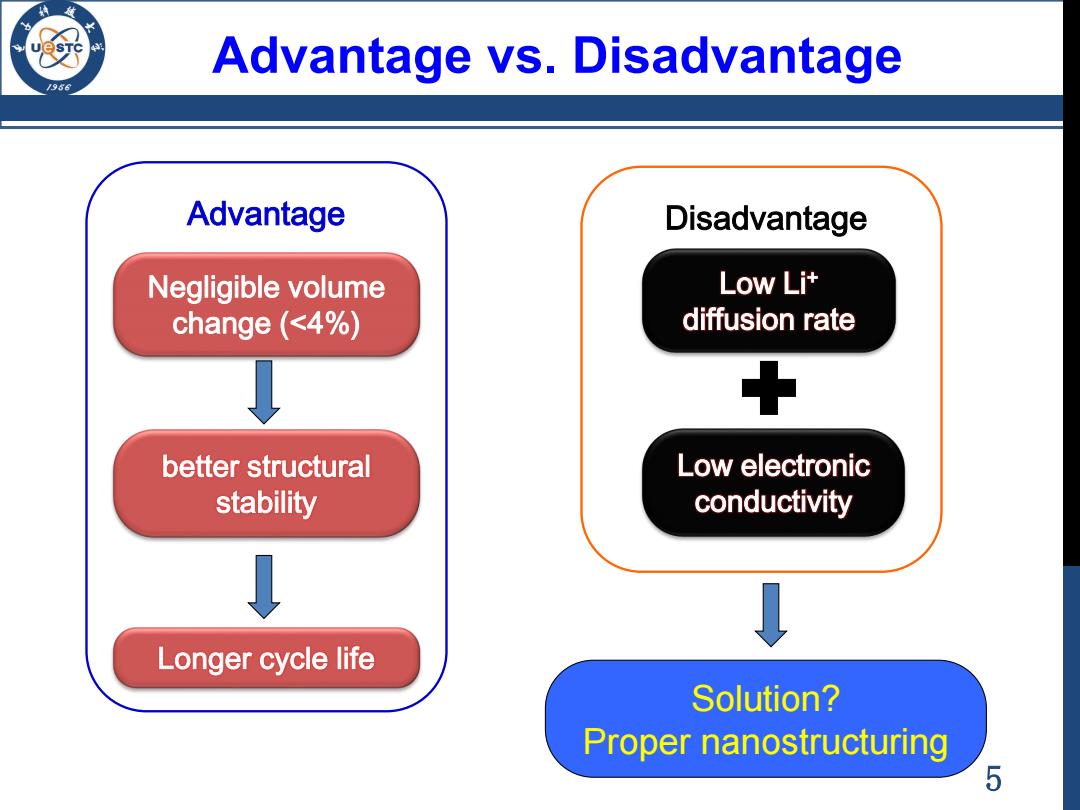
Advantage vs.Disadvantage 956 Advantage Disadvantage Negligible volume Low Li+ change (<4%) diffusion rate better structural Low electronic stability conductivity Longer cycle life Solution? Proper nanostructuring 5
5 Advantage vs. Disadvantage Negligible volume change (<4%) better structural stability Longer cycle life Advantage Solution? Proper nanostructuring Low Li+ diffusion rate Low electronic conductivity Disadvantage
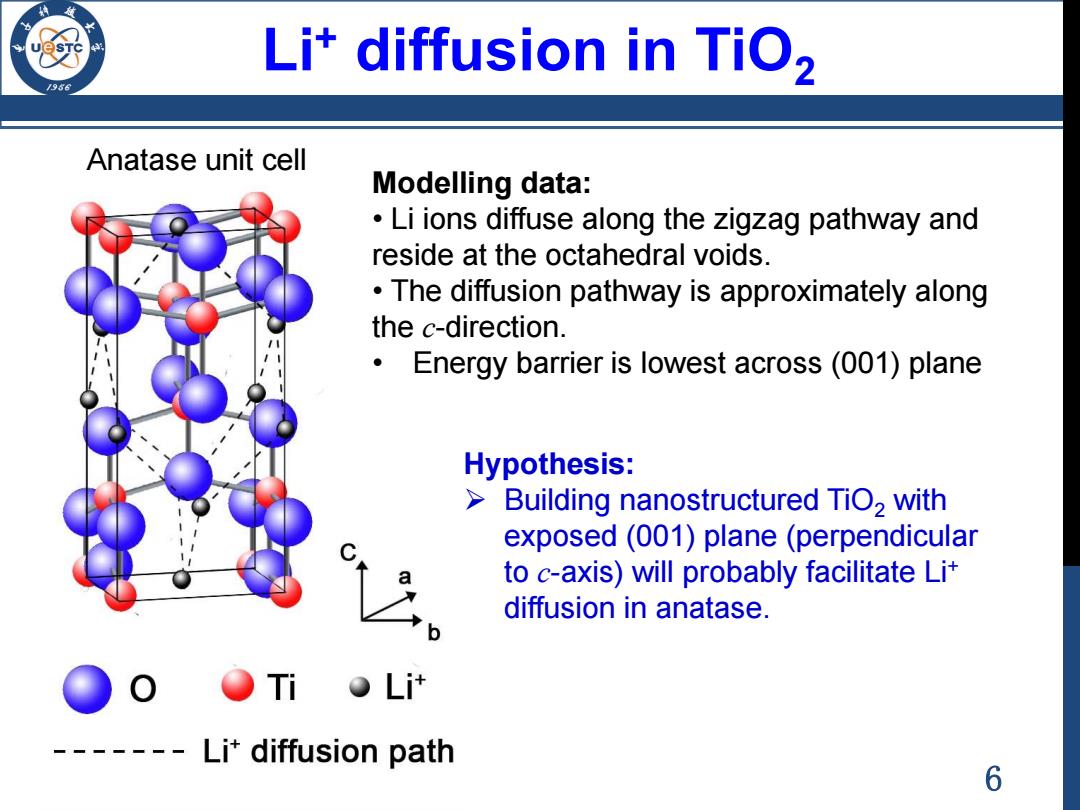
Li*diffusion in TiO2 Anatase unit cell Modelling data: Li ions diffuse along the zigzag pathway and reside at the octahedral voids. The diffusion pathway is approximately along the c-direction. Energy barrier is lowest across (001)plane Hypothesis: Building nanostructured TiO2 with exposed(001)plane(perpendicular a to c-axis)will probably facilitate Lit diffusion in anatase. O Ti L计 Li'diffusion path 6
6 Anatase unit cell Modelling data: • Li ions diffuse along the zigzag pathway and reside at the octahedral voids. • The diffusion pathway is approximately along the c-direction. • Energy barrier is lowest across (001) plane Hypothesis: Building nanostructured TiO2 with exposed (001) plane (perpendicular to c-axis) will probably facilitate Li+ diffusion in anatase. Li+ diffusion in TiO2

(001)Facet of Anatase TiO2 LETTERS Anatase TiOz single crystals with a large percentage of reactive facets Hua Gui Yang'*,Cheng Hua Sun.2*,Shi Zhang Qiao',Jin Zou3,Gang Liu.4,Sean Campbell Smith2, Hui Ming Cheng&Gao Qing Lu much more reactive (001}facets-13,18-20.Here we demonstrate that for fluorine-terminated surfaces this relative stability is reversed: (001}is energetically preferable to {101).We explored this effect systematically for a range of non-metallic adsorbate atoms by first-principle quantum chemical calculations.On the basis of theoretical predictions,we have synthesized uniform anatase TiO2 single crystals with a high percentage(47 per cent)of (001} facets using hydrofluoric acid as a morphology controlling agent. Moreover,the fluorated surface of anatase single crystals can easily be cleaned using heat treatment to render a fluorine-free surface Hua Gui YANG without altering the crystal structure and morphology
(001) Facet of Anatase TiO2 Hua Gui YANG
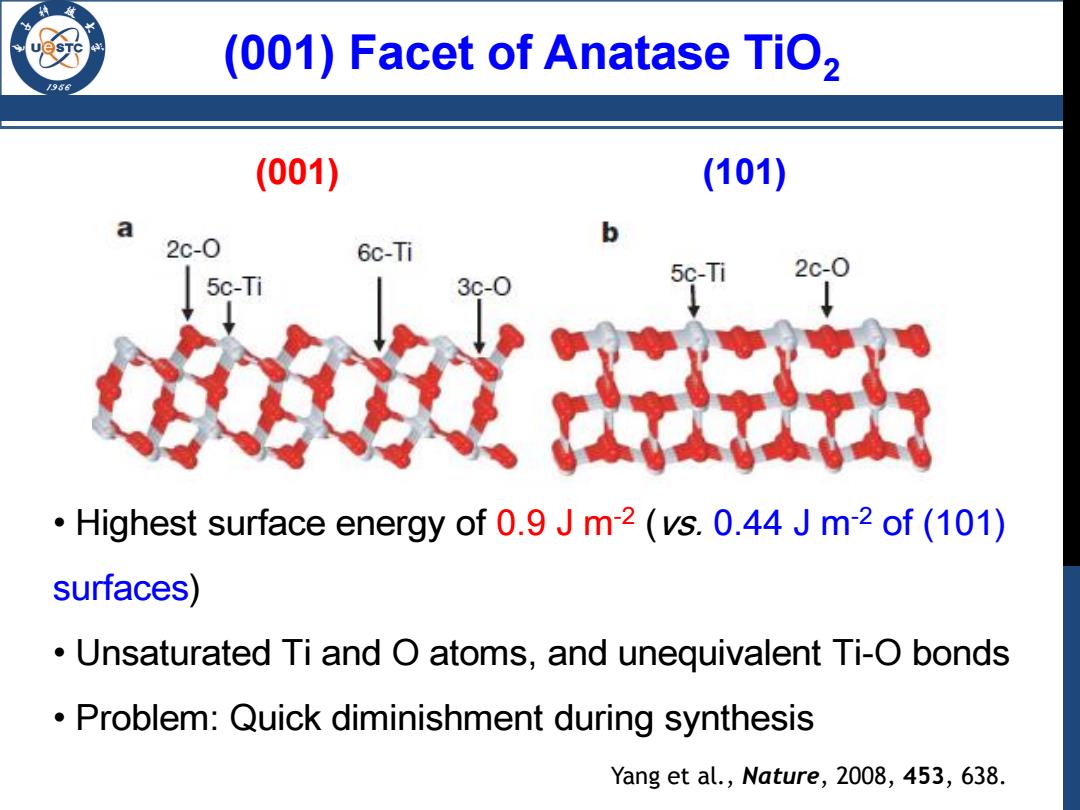
(001)Facet of Anatase TiO, /98 (001) (101) a b 2c-0 6c-Ti 5c-Ti 2c-0 5c-Ti 3c-0 Highest surface energy of 0.9 J m2 (vs.0.44 J m-2 of (101) surfaces) Unsaturated Ti and O atoms,and unequivalent Ti-O bonds Problem:Quick diminishment during synthesis Yang et al.,Nature,2008,453,638
• Highest surface energy of 0.9 J m-2 (vs. 0.44 J m-2 of (101) surfaces) • Unsaturated Ti and O atoms, and unequivalent Ti-O bonds • Problem: Quick diminishment during synthesis (001) (101) (001) Facet of Anatase TiO2 Yang et al., Nature, 2008, 453, 638
Analyze surface stabilizing agents 1/936 (001) (101)
Analyze surface stabilizing agents (001) (101)

Effect of different atoms 001 B Fluorine(F)as a stabilizing agent (101) A >Binds strongly to Ti >Significantly lowers the surface energy Preserve(001)facets 1.2 8 001 B/A 0-101 6 0.9 -D-Sco:/S 4 oney 0.6 2 0.3 0.0 口中0中0 -2clean HBCNOF SIPS CIBr I Clean HB C NO F SiP S CIBr I X X
Effect of different atoms Fluorine (F) as a stabilizing agent Binds strongly to Ti Significantly lowers the surface energy Preserve (001) facets