
Lecture 5 1956 Cathode material for LIBs: Li-Mn-O and NCM Chen Junsong School of Materials and Energy 2020.04
Cathode material for LIBs: Li-Mn-O and NCM Chen Junsong School of Materials and Energy 2020.04 Lecture 5

Content /986 ·Introduction 00 Chemistry Molecular structure and Li diffusion Advantages and disadvantages 2
Content • Introduction • Chemistry • Molecular structure and Li diffusion • Advantages and disadvantages 2

Phase diagram of Li-Mn-O /986 Mn Multiple compositions Due to different oxidation states of Mn Il-VII Most important states Il,IV,VIl MnO LiMngO Most stable state ll MnO LizMn,O LiMnO2☑ λ-MnO2 LiMn,On2 λ-MnO2 Li,O LiMn204 4V Solid State lonics,69(1994)59 LiMnO2 3V Li2Mn4O9 LizMns1 LiMngO12 LisMn409 3V Li2MnO3∠天Li6.5Mn5O12 3
Phase diagram of Li-Mn-O • Multiple compositions • Due to different oxidation states of Mn II-VII • Most important states II, IV, VII • Most stable state II Solid State Ionics, 69 (1994) 59 3
Types of Li-Mn-O /986 ·LiMn2O4 。Spinel structure LiMnO2 Layered structure,like LiCoO, 4
Types of Li-Mn-O • LiMn2O4 • Spinel structure • LiMnO2 • Layered structure, like LiCoO2 4
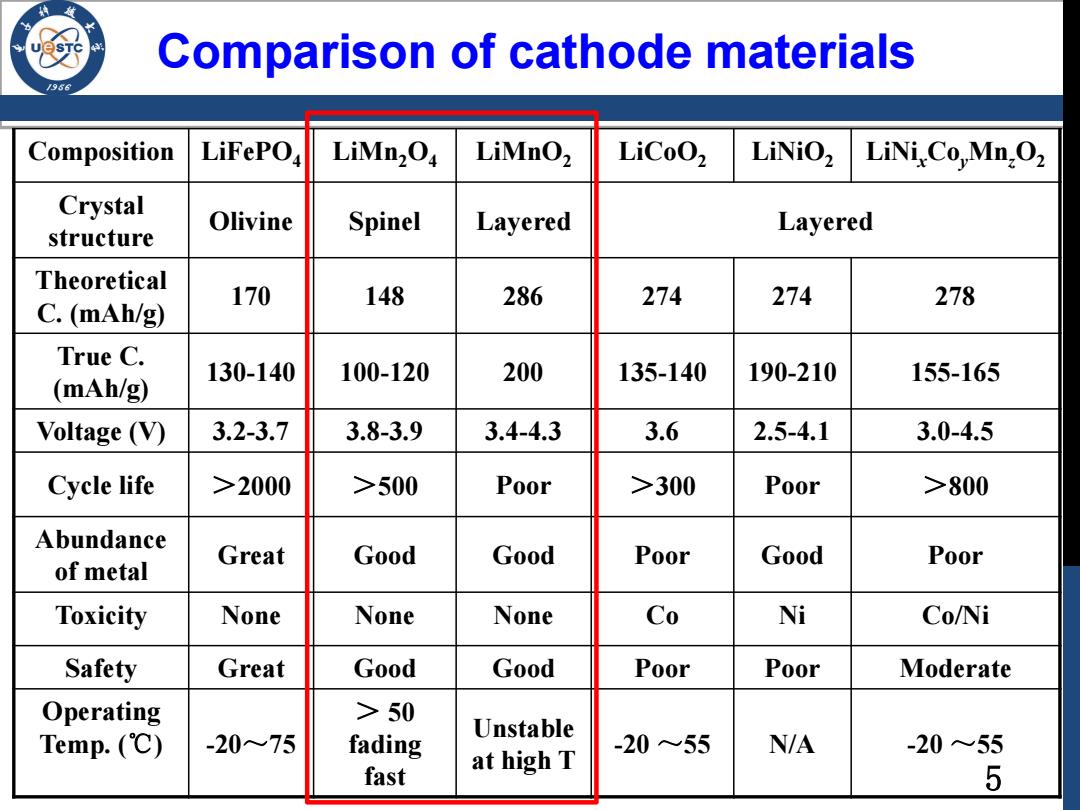
Comparison of cathode materials Composition LiFePOa LiMn2O LiMnO2 LiCoO2 LiNiOz LiNiCo,Mn,O2 Crystal Olivine Spinel Layered structure Layered Theoretical C.(mAh/g) 170 148 286 274 274 278 True C. 130-140 100-120 200 135-140 190-210 155-165 (mAh/g) Voltage(V) 3.2-3.7 3.8-3.9 3.4-4.3 3.6 2.5-4.1 3.0-4.5 Cycle life >2000 >500 Poor >300 Poor >800 Abundance Great Good Good Poor Good Poor of metal Toxicity None None None Co Ni Co/Ni Safety Great Good Good Poor Poor Moderate Operating >50 Temp.(℃) -20~75 fading Unstable -20~55 N/A -20~55 fast at high T 5
Comparison of cathode materials Composition LiFePO4 LiMn2O4 LiMnO2 LiCoO2 LiNiO2 LiNixCoyMnzO2 Crystal structure Olivine Spinel Layered Layered Theoretical C. (mAh/g) 170 148 286 274 274 278 True C. (mAh/g) 130-140 100-120 200 135-140 190-210 155-165 Voltage (V) 3.2-3.7 3.8-3.9 3.4-4.3 3.6 2.5-4.1 3.0-4.5 Cycle life >2000 >500 Poor >300 Poor >800 Abundance of metal Great Good Good Poor Good Poor Toxicity None None None Co Ni Co/Ni Safety Great Good Good Poor Poor Moderate Operating Temp. (℃) -20~75 > 50 fading fast Unstable at high T -20 ~55 N/A -20 ~55 5

4 1956 Layered LiMnO2 6
Layered LiMnO2 6

Layered LiMnO2 /986 Synthesis of layered LiMnO,as an electrode for rechargeable lithium batteries A.Robert Armstrong Peter G.Bruce School of Chemistry,University of St Andrews,North Haugh, St Andrews,Fife KY16 9ST,UK Peter G.Bruce Nature volume381,pages499-500(06 June 1996) Here we report the synthesis and electrochemical performance of a new material,layered LiMnO2,which is structurally analogous to LiCoO2.The charge capacity of LiMnO2(~270 mAhg-1)compares well with that of both LiCoO,and LiMn2O4,and preliminary results indicate good stability over repeated charge-discharge cycles. 7
Layered LiMnO2 Nature volume381, pages499–500 (06 June 1996) Here we report the synthesis and electrochemical performance of a new material, layered LiMnO2, which is structurally analogous to LiCoO2. The charge capacity of LiMnO2 (∼270 mAhg–1) compares well with that of both LiCoO2 and LiMn2O4, and preliminary results indicate good stability over repeated charge–discharge cycles. Peter G. Bruce 7
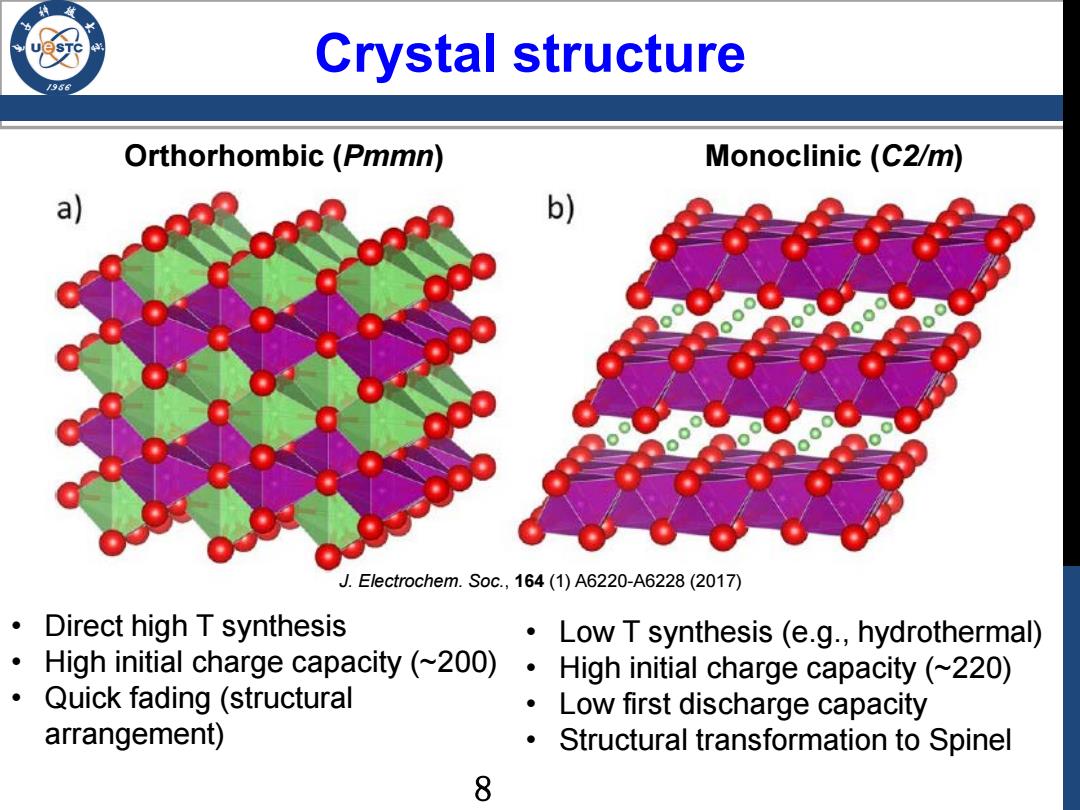
Crystal structure Orthorhombic (Pmmn) Monoclinic (C2/m) a b) J.Electrochem.Soc.,164(1)A6220-A6228(2017) Direct high T synthesis Low T synthesis (e.g.,hydrothermal) High initial charge capacity (~200) High initial charge capacity(~220) Quick fading (structural Low first discharge capacity arrangement) Structural transformation to Spinel 8
Crystal structure Orthorhombic (Pmmn) Monoclinic (C2/m) • Direct high T synthesis • High initial charge capacity (~200) • Quick fading (structural arrangement) • Low T synthesis (e.g., hydrothermal) • High initial charge capacity (~220) • Low first discharge capacity • Structural transformation to Spinel J. Electrochem. Soc., 164 (1) A6220-A6228 (2017) 8

Theoretical capacity /98 At the cathode: LiMnO2(Mn3+)←→MnO2(Mn4+)+Lit+e Molecular weight of: Li:6.94 g/mol,Mn:54.94 g/mol,O:16 g/mol So,molecular weight of LiMnO2: 6.94+54.94+16×2=93.88g/mol So,the theoretical capacity is: 96500÷93.88÷3.6=285.53mAh/g 9
Theoretical capacity At the cathode: LiMnO2 (Mn3+) ↔ MnO2 (Mn4+)+ Li+ + e─ Molecular weight of: Li: 6.94 g/mol, Mn: 54.94 g/mol, O: 16 g/mol So, molecular weight of LiMnO2: 6.94 + 54.94 + 16 × 2 = 93.88 g/mol So, the theoretical capacity is: 96500÷93.88÷3.6 = 285.53 mA·h/g 9
Drawbacks of LiMnO2 Structural instability:transform from layered to spinel structure upon Li extraction > Vitins and West (J Electrochem.Soc.,Vol.144,No.8, 1997): Mn with high movability-rearrangement of structure; Very low reversible capacity >Jahn-Teller (J-T)distortion: The geometrical distortion of molecules and ions that is associated with certain electron configurations.(Wikipedia) ·Mn3+isJ-T active 10
Drawbacks of LiMnO2 Vitins and West (J Electrochem. Soc., Vol. 144, No. 8, 1997): • Mn with high movability → rearrangement of structure; • Very low reversible capacity Jahn-Teller (J-T) distortion: • The geometrical distortion of molecules and ions that is associated with certain electron configurations. (Wikipedia) • Mn3+ is J-T active Structural instability: transform from layered to spinel structure upon Li extraction 10