正在加载图片...
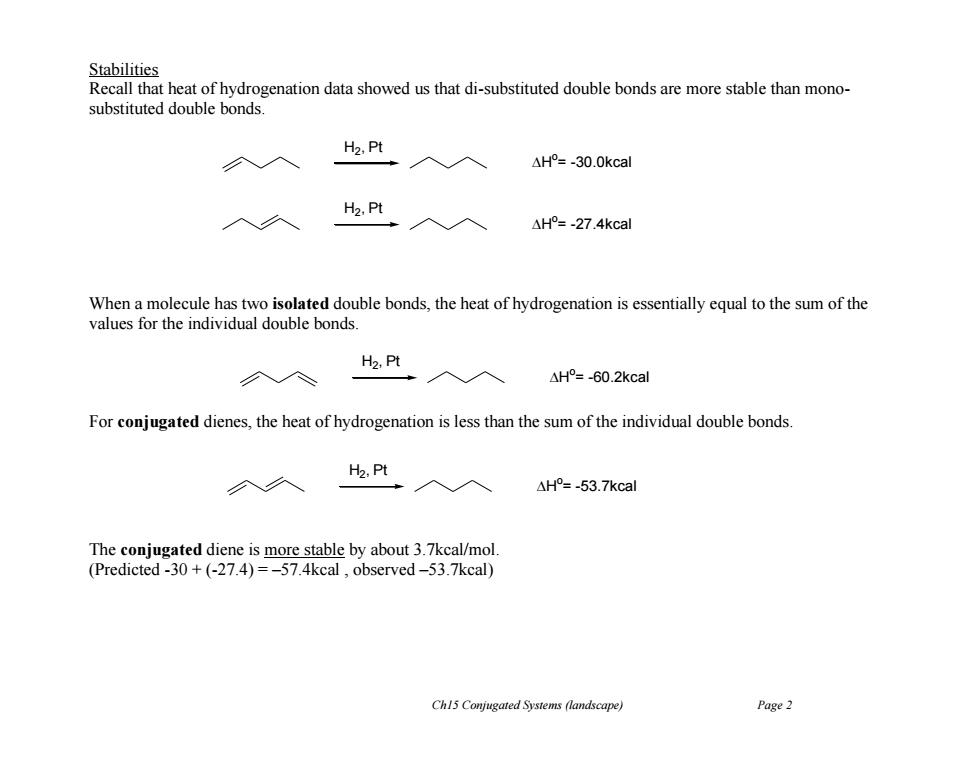
Stabilities Recall that heat of hydrogenation data showed us that di-substituted double bonds are more stable than mono- substituted double bonds. H2,Pt 入入 △H°=-30.0kcal H2,Pt △H°=-27.4kcal When a molecule has two isolated double bonds,the heat of hydrogenation is essentially equal to the sum of the values for the individual double bonds H2,Pt 个◇ 入入 AH°=-60.2kcal For conjugated dienes,the heat of hydrogenation is less than the sum of the individual double bonds. H2,Pt ·入入 △H°=-53.7kcal The conjugated diene is more stable by about 3.7kcal/mol. (Predicted-30+(-27.4)=-57.4kcal,observed-53.7kcal) Ch15 Conjugated Systems (landscape) Page 2Ch15 Conjugated Systems (landscape) Page 2 Stabilities Recall that heat of hydrogenation data showed us that di-substituted double bonds are more stable than monosubstituted double bonds. When a molecule has two isolated double bonds, the heat of hydrogenation is essentially equal to the sum of the values for the individual double bonds. For conjugated dienes, the heat of hydrogenation is less than the sum of the individual double bonds. The conjugated diene is more stable by about 3.7kcal/mol. (Predicted -30 + (-27.4) = –57.4kcal , observed –53.7kcal) H2 , Pt H o = -30.0kcal H2 , Pt H o = -27.4kcal H2 , Pt H o = -60.2kcal H2 , Pt H o = -53.7kcal