正在加载图片...
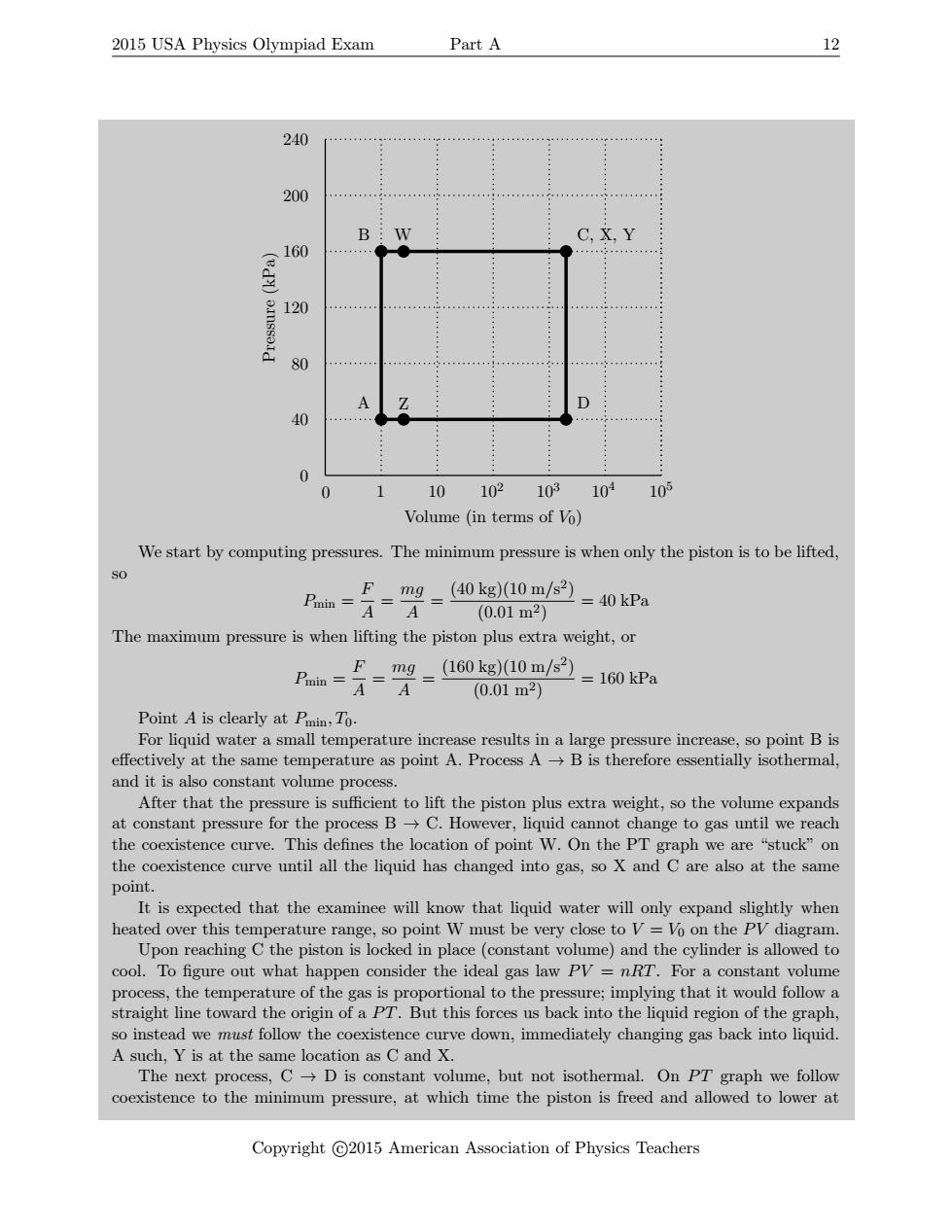
2015 USA Physics Olympiad Exam Part A 12 240 200 …………… B C.X.Y 160 ed) 120 4444 444444 80 A 40 0 0 1 10 102 103 101 105 Volume (in terms of Vo) We start by computing pressures.The minimum pressure is when only the piston is to be lifted, SO F=m9-40kg10m/s) =40 kPa (0.01m2) The maximum pressure is when lifting the piston plus extra weight,or Fmg 160kg10m/)=160kPa (0.01m2) Point A is clearly at Pmin,To. For liquid water a small temperature increase results in a large pressure increase,so point B is effectively at the same temperature as point A.Process A->B is therefore essentially isothermal, and it is also constant volume process. After that the pressure is sufficient to lift the piston plus extra weight,so the volume expands at constant pressure for the process BC.However,liquid cannot change to gas until we reach the coexistence curve.This defines the location of point W.On the PT graph we are "stuck"on the coexistence curve until all the liquid has changed into gas,so X and C are also at the same point. It is expected that the examinee will know that liquid water will only expand slightly when heated over this temperature range,so point W must be very close to V=Vo on the PV diagram. Upon reaching C the piston is locked in place (constant volume)and the cylinder is allowed to cool.To figure out what happen consider the ideal gas law PV =nRT.For a constant volume process,the temperature of the gas is proportional to the pressure;implying that it would follow a straight line toward the origin of a PT.But this forces us back into the liquid region of the graph, so instead we must follow the coexistence curve down,immediately changing gas back into liquid. A such,Y is at the same location as C and X. The next process,CD is constant volume,but not isothermal.On PT graph we follow coexistence to the minimum pressure,at which time the piston is freed and allowed to lower at Copyright C2015 American Association of Physics Teachers2015 USA Physics Olympiad Exam Part A 12 0 1 10 102 103 104 105 0 40 80 120 160 200 240 Volume (in terms of V0) Pressure (kPa) A B C, X, Y D W Z We start by computing pressures. The minimum pressure is when only the piston is to be lifted, so Pmin = F A = mg A = (40 kg)(10 m/s 2 ) (0.01 m2) = 40 kPa The maximum pressure is when lifting the piston plus extra weight, or Pmin = F A = mg A = (160 kg)(10 m/s 2 ) (0.01 m2) = 160 kPa Point A is clearly at Pmin, T0. For liquid water a small temperature increase results in a large pressure increase, so point B is effectively at the same temperature as point A. Process A → B is therefore essentially isothermal, and it is also constant volume process. After that the pressure is sufficient to lift the piston plus extra weight, so the volume expands at constant pressure for the process B → C. However, liquid cannot change to gas until we reach the coexistence curve. This defines the location of point W. On the PT graph we are “stuck” on the coexistence curve until all the liquid has changed into gas, so X and C are also at the same point. It is expected that the examinee will know that liquid water will only expand slightly when heated over this temperature range, so point W must be very close to V = V0 on the P V diagram. Upon reaching C the piston is locked in place (constant volume) and the cylinder is allowed to cool. To figure out what happen consider the ideal gas law P V = nRT. For a constant volume process, the temperature of the gas is proportional to the pressure; implying that it would follow a straight line toward the origin of a P T. But this forces us back into the liquid region of the graph, so instead we must follow the coexistence curve down, immediately changing gas back into liquid. A such, Y is at the same location as C and X. The next process, C → D is constant volume, but not isothermal. On P T graph we follow coexistence to the minimum pressure, at which time the piston is freed and allowed to lower at Copyright c 2015 American Association of Physics Teachers