正在加载图片...
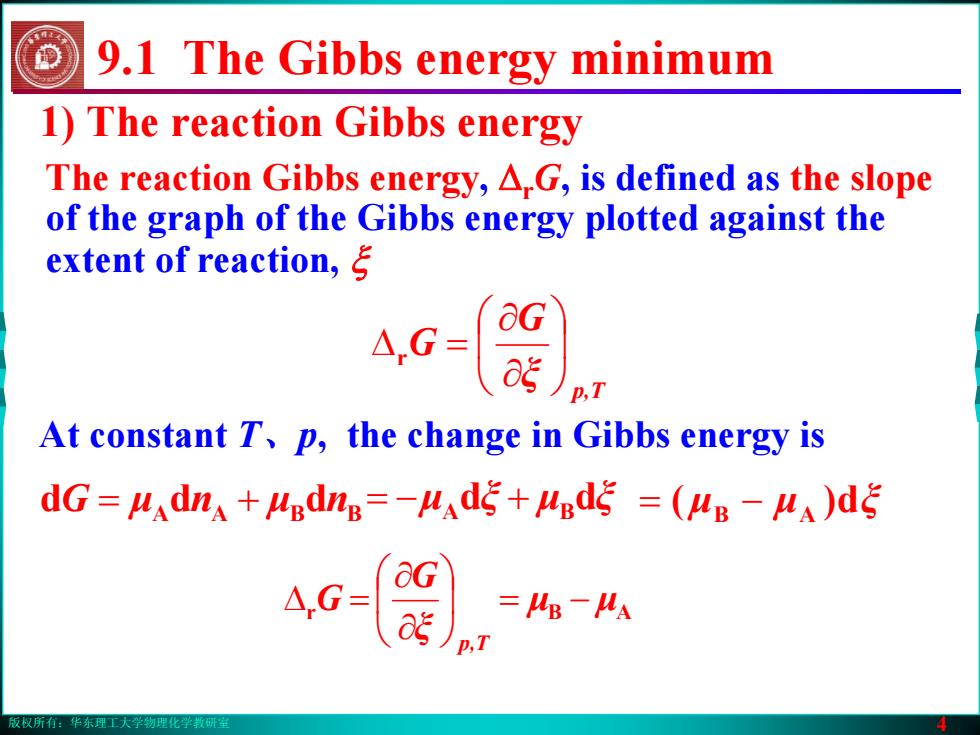
版权所有:华东理工大学物理化学教研室 4 1) The reaction Gibbs energy The reaction Gibbs energy, ΔrG, is defined as the slope of the graph of the Gibbs energy plotted against the extent of reaction, ξ p,T ξ G G ⎟⎠⎞ ⎜⎝⎛ ∂∂ r =Δ At constant T、p, the change in Gibbs energy is G = μ n + μ ddd nBBAA = − A + Bdd ξμξμ = − AB )d( ξμμ r μμ AB ξ G G p,T ⎟ −= ⎠⎞ ⎜⎝⎛ ∂∂ =Δ 9.1 The Gibbs energy minimum版权所有:华东理工大学物理化学教研室 4 1) The reaction Gibbs energy The reaction Gibbs energy, ΔrG, is defined as the slope of the graph of the Gibbs energy plotted against the extent of reaction, ξ p,T ξ G G ⎟⎠⎞ ⎜⎝⎛ ∂∂ r =Δ At constant T、p, the change in Gibbs energy is G = μ n + μ ddd nBBAA = − A + Bdd ξμξμ = − AB )d( ξμμ r μμ AB ξ G G p,T ⎟ −= ⎠⎞ ⎜⎝⎛ ∂∂ =Δ 9.1 The Gibbs energy minimum