正在加载图片...
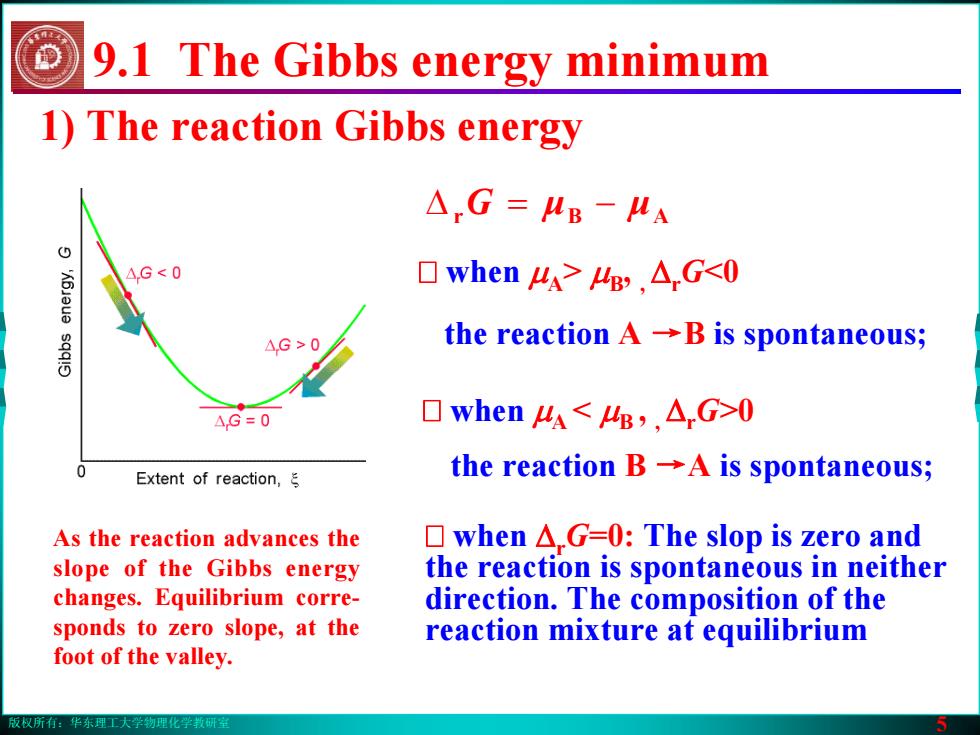
版权所有:华东理工大学物理化学教研室 5 1) The reaction Gibbs energy As the reaction advances the slope of the Gibbs energy changes. Equilibrium corresponds to zero slope, at the foot of the valley. when μA> μB, , ΔrG<0 the reaction A →B is spontaneous; when μA < μB , , ΔrG>0 the reaction B →A is spontaneous; ΔrG = − μμ AB when ΔrG=0: The slop is zero and the reaction is spontaneous in neither direction. The composition of the reaction mixture at equilibrium 9.1 The Gibbs energy minimum版权所有:华东理工大学物理化学教研室 5 1) The reaction Gibbs energy As the reaction advances the slope of the Gibbs energy changes. Equilibrium corresponds to zero slope, at the foot of the valley. when μA> μB, , ΔrG<0 the reaction A →B is spontaneous; when μA < μB , , ΔrG>0 the reaction B →A is spontaneous; ΔrG = − μμ AB when ΔrG=0: The slop is zero and the reaction is spontaneous in neither direction. The composition of the reaction mixture at equilibrium 9.1 The Gibbs energy minimum