正在加载图片...
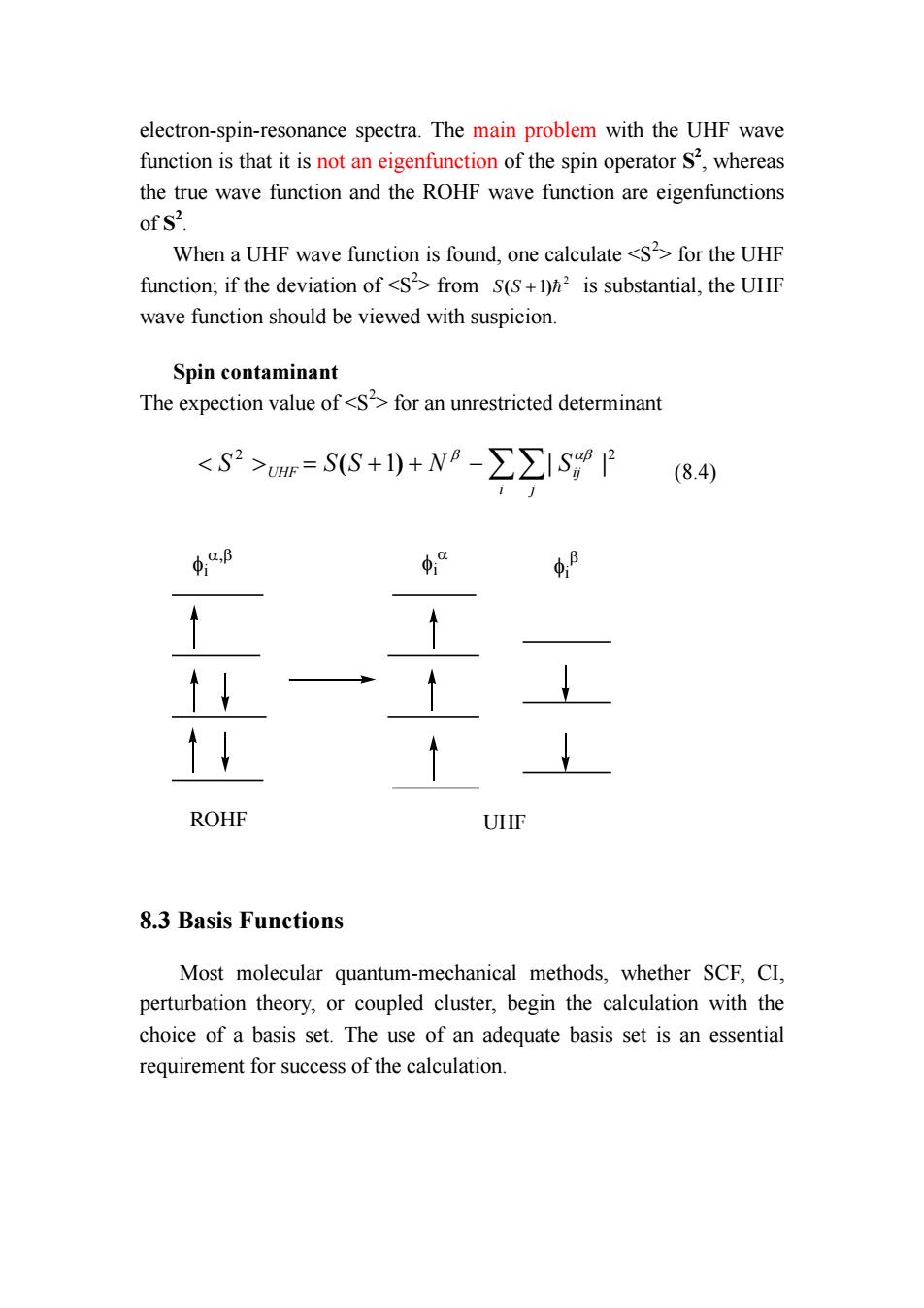
electron-spin-resonance spectra.The main problem with the UHF wave function is that it is not an eigenfunction of the spin operator S2,whereas the true wave function and the ROHF wave function are eigenfunctions of S2. When a UHF wave function is found,one calculate <S2>for the UHF function;if the deviation of <S2>from S(S+)h is substantial,the UHF wave function should be viewed with suspicion Spin contaminant The expection value of<Sfor an unrestricted determinant <S2>=SS+1)+N8-∑∑ISP84 4.B φ“ ↑↓ ROHF UHF 8.3 Basis Functions Most molecular quantum-mechanical methods,whether SCF,CI, perturbation theory,or coupled cluster,begin the calculation with the choice of a basis set.The use of an adequate basis set is an essential requirement for success of the calculation.electron-spin-resonance spectra. The main problem with the UHF wave function is that it is not an eigenfunction of the spin operator S2 , whereas the true wave function and the ROHF wave function are eigenfunctions of S2 . When a UHF wave function is found, one calculate <S2 > for the UHF function; if the deviation of <S2 > from 2 S(S +1)= is substantial, the UHF wave function should be viewed with suspicion. Spin contaminant The expection value of <S2 > for an unrestricted determinant 2 2 < > = ( +1) + −∑∑| | i j S UHF S S N Sij β αβ (8.4) ROHF UHF φi α,β φi α φi β 8.3 Basis Functions Most molecular quantum-mechanical methods, whether SCF, CI, perturbation theory, or coupled cluster, begin the calculation with the choice of a basis set. The use of an adequate basis set is an essential requirement for success of the calculation