
Chapter 8 Ab Initio Treatments of Polyatomic Molecules 8.1 Ab Initio Methods andand Semiemprical Methods Semiempirical and Ab initio Methods Molecular quantum-mechanical methods are classified as either ab initio or semiempirical. Semiempirical methods use a simple Hamiltonian than the correct molecular Hamiltionian and use parameters whose values are adjusted to fit experimental data or the results of ab initio calculations. HMO,EHMO,CNDO,... An ab initio calculation uses the correct Hamiltonian and does not use experimental data other than the values of the fundamental physical constants.(Ab initio is Latin for"from the beginning"and indicates a calculation based on fundamental principles.) Hartree-Fock SCF calculation: Basis set→Atomic orbital→Molecular orbital→Slater determinant →Iterative solution. Classification of Electronic Terms of Polyatomic Molecules Linear Molecules: The operator L:for the axial component of the total electronic orbital momentum commutes with the electronic Hamitionian,and the same classifications are used as for diatomic molecules;we have such possibilities as'∑*,l∑,3Σ,l'L,and so on.For linear polyatomic molecules with a center of symmetry,the g.u classification is added. Nonlinear polyatomic molecules: There is no orbital angular-momentum operator that commutes with the electronic Hamiltionian,and the angular momentum classification of electronic terms cannot be used.Operators that do commute with the electronic Hamiltionian are the symmetry operators OR of the molecule. and the electronic states of polyatomic molecules are classified according
Chapter 8 Ab Initio Treatments of Polyatomic Molecules 8.1 Ab Initio Methods and and Semiemprical Methods Semiempirical and Ab initio Methods Molecular quantum-mechanical methods are classified as either ab initio or semiempirical. Semiempirical methods use a simple Hamiltonian than the correct molecular Hamiltionian and use parameters whose values are adjusted to fit experimental data or the results of ab initio calculations. HMO, EHMO, CNDO, … An ab initio calculation uses the correct Hamiltonian and does not use experimental data other than the values of the fundamental physical constants. (Ab initio is Latin for “from the beginning” and indicates a calculation based on fundamental principles.) Hartree-Fock SCF calculation: Basis set → Atomic orbital → Molecular orbital → Slater determinant → Iterative solution … Classification of Electronic Terms of Polyatomic Molecules Linear Molecules: The operator Lz for the axial component of the total electronic orbital momentum commutes with the electronic Hamitionian, and the same classifications are used as for diatomic molecules; we have such possibilities as 1 Σ+ , 1 Σ- , 3 Σ+ , 1 Π, and so on. For linear polyatomic molecules with a center of symmetry, the g, u classification is added. Nonlinear polyatomic molecules: There is no orbital angular-momentum operator that commutes with the electronic Hamiltionian, and the angular momentum classification of electronic terms cannot be used. Operators that do commute with the electronic Hamiltionian are the symmetry operators OR of the molecule, and the electronic states of polyatomic molecules are classified according

to the behavior of the electronic wave function on application of these operators Consider H2O as an example. In its equilibrium configuration,water belongs to group Car with the symmetry operators EC2(z)o,(Xz)oyz) E C2(z)(xZ)(yz) N 11 1 1 1 1 -1 1 -1 1 -1 B2 -1 -1 1 Consider an operator R that commutes with the molecular Hamiltonian H that does not involve spin;we have RH=HR RHΨ=REΨ HR)=E(RΨ) (8.1) so that R'is an eigenfunction of H with eigenvalue E.We have RΨ=λΨ (8.2) Here must be an eigenfunction of the symmetry operator R.Thus,the electronic states of polyatomic molecules can be classified according to the symmetry of the electronic wave function associated with the molecular point group.For the term classification of H2O,we have such possibilities as A,A2.B,B2,A,etc. As an example,the possible symmetry species of a Dh molecule to be
to the behavior of the electronic wave function on application of these operators. Consider H2O as an example. In its equilibrium configuration, water belongs to group C2V with the symmetry operators E C2(z) σv(xz) σv(yz) H1 H2 O y z E C2(z) σv(xz) σv(yz) A1 A2 B1 B2 1 1 1 1 1 1 1 1 -1 -1 -1 1 -1 -1 -1 1 Consider an operator R that commutes with the molecular Hamiltonian H that does not involve spin; we have RH = HR RHΨ = REΨ H(RΨ) = E(RΨ) (8.1) so that RΨ is an eigenfunction of H with eigenvalue E. We have RΨ = λΨ (8.2) Here Ψ must be an eigenfunction of the symmetry operator R. Thus, the electronic states of polyatomic molecules can be classified according to the symmetry of the electronic wave function associated with the molecular point group. For the term classification of H2O, we have such possibilities as 1 A1, 1A2, 1 B1, 1 B2, 3 A1, etc. As an example, the possible symmetry species of a D6h molecule to be

Alg,A2g Big,B2g,Eig E2g Alu A2u Blu B2us Elu E2u 8.2 The SCF MO Treatment of Polyatomic Molecules The purely electronic nonrelativistic Hamiltonian for a polyatomic molecule is(in atomic units): 户=-Σ:-∑Σ2+2Σ1 (8.3) Best possible variation function-Hartree-Fock SCF function:The form of an antisymmetrized product of spin-orbitals.The MOs are usually expressed as linear combinations of basis functions,the coefficients being found by solution of the Roothaan equations. A sufficiently large basis set-Accurate approximations to the Hartree-Fock MO's. A minimal basis set->only rough approximations to the Hartree-Fock MO's,still referred to as SCF molecular orbitals. Classification of Molecular Orbitals As might be expected,the MOs of a polyatomic molecule show the same kinks of possible symmetry behavior as the electronic wavefunction does.The MOs are therefore classified according to the symmetry species of the molecular point group. For example,the MOs of H2O have the possible symmetry species a,a2, b1,and b2.Lowercase letters are used for MO symmetry species.To distinguish MOs of the same symmetry species,we number them in order of increasing energy.1a,2a,3a,1b,2b1,. For benzene C6H6,there are some doubly degenerate symmetry species, so some of the benzene MOs occur in having the same energy. Specification of the number of electrons in each MO specifies the
A1g, A2g, B1g, B2g, E1g, E2g A1u, A2u, B1u, B2u, E1u, E2u 8.2 The SCF MO Treatment of Polyatomic Molecules The purely electronic nonrelativistic Hamiltonian for a polyatomic molecule is (in atomic units): ∑ ∑∑ ∑∑ > ∧ = − ∇ − + i i i i i j ij i el r r Z H 1 2 1 2 α α α (8.3) Best possible variation function — Hartree-Fock SCF function: The form of an antisymmetrized product of spin-orbitals. The MOs are usually expressed as linear combinations of basis functions, the coefficients being found by solution of the Roothaan equations. A sufficiently large basis set → Accurate approximations to the Hartree-Fock MO’s. A minimal basis set → only rough approximations to the Hartree-Fock MO’s, still referred to as SCF molecular orbitals. Classification of Molecular Orbitals As might be expected, the MOs of a polyatomic molecule show the same kinks of possible symmetry behavior as the electronic wavefunction does. The MOs are therefore classified according to the symmetry species of the molecular point group. For example, the MOs of H2O have the possible symmetry species a1, a2, b1, and b2. Lowercase letters are used for MO symmetry species. To distinguish MOs of the same symmetry species, we number them in order of increasing energy. 1a1, 2a1, 3a1, …; 1b1, 2b1, … For benzene C6H6, there are some doubly degenerate symmetry species, so some of the benzene MOs occur in having the same energy. Specification of the number of electrons in each MO specifies the

molecular electronic configuration.For example,an (eg)configuration of Doh molecule gives the termsAg E2g,andA2g. A closed-shell configuration gives rise to a single nondegenerate term whose multiplicity is I and whose symmetry species is the totally symmetric. SCF-MO Wave Functions for Open-Shell States. For SCF MO calculations of closed-shell states of molecules and atoms,electrons paired with each other almost given precisely the same spatial orbital function.A Hartree-Fock wave function in which electrons whose spins are paired occupy the same spatial orbital is called a restricted Hartree-Fock(RHF)wave function For open-shell states,two different approaches are widely used. Restricted Open-Shell Hartree-Fock(ROHF)Method In the ROHF method,electrons that are paired with each other are given the same spatial orbital function.For example,the ROHF wave function of the Li ground state is |sls2s1,where the two Is electrons occupy the same spatial MO.The 2s electron in this ROHF function has been given spin a. Restricted Open-Shell Hartree-Fock(ROHF)Method Since electron with the same spin tend to keep away from each other (Pauli repulsion),the interaction between the 2sa and Isa electrons differs from the interaction between the 2sa and IsB electrons,and it seems reasonable to give the two Is electrons slightly different spatial orbitals,which we call Is and Is'.This gives the unrestricted Hartree-Fock (UHF)wave function for the Li ground state as |1s1s'2s, where Is Is'.In a UHF wave function,the spatial orbitals of spin-a electrons are allowed to differ from those of spin-B electrons Comparison of ROHF with UHF The UHF wave function gives a slightly lower energy than the ROHF wave function and is much more useful in predicting
molecular electronic configuration. For example, an (e1g) 2 configuration of D6h molecule gives the terms 1 A1g, 1 E2g, and 3 A2g. A closed-shell configuration gives rise to a single nondegenerate term whose multiplicity is 1 and whose symmetry species is the totally symmetric. SCF-MO Wave Functions for Open-Shell States. For SCF MO calculations of closed-shell states of molecules and atoms, electrons paired with each other almost given precisely the same spatial orbital function. A Hartree-Fock wave function in which electrons whose spins are paired occupy the same spatial orbital is called a restricted Hartree-Fock (RHF) wave function. For open-shell states, two different approaches are widely used. Restricted Open-Shell Hartree-Fock (ROHF) Method In the ROHF method, electrons that are paired with each other are given the same spatial orbital function. For example, the ROHF wave function of the Li ground state is |1s1s2s |, where the two 1s electrons occupy the same spatial MO. The 2s electron in this ROHF function has been given spin α. Restricted Open-Shell Hartree-Fock (ROHF) Method Since electron with the same spin tend to keep away from each other (Pauli repulsion), the interaction between the 2sα and 1sα electrons differs from the interaction between the 2sα and 1sβ electrons, and it seems reasonable to give the two 1s electrons slightly different spatial orbitals, which we call 1s and 1s′. This gives the unrestricted Hartree-Fock (UHF) wave function for the Li ground state as |1s1s'2s | , where 1s ≠ 1s′. In a UHF wave function, the spatial orbitals of spin-α electrons are allowed to differ from those of spin-β electrons. Comparison of ROHF with UHF The UHF wave function gives a slightly lower energy than the ROHF wave function and is much more useful in predicting
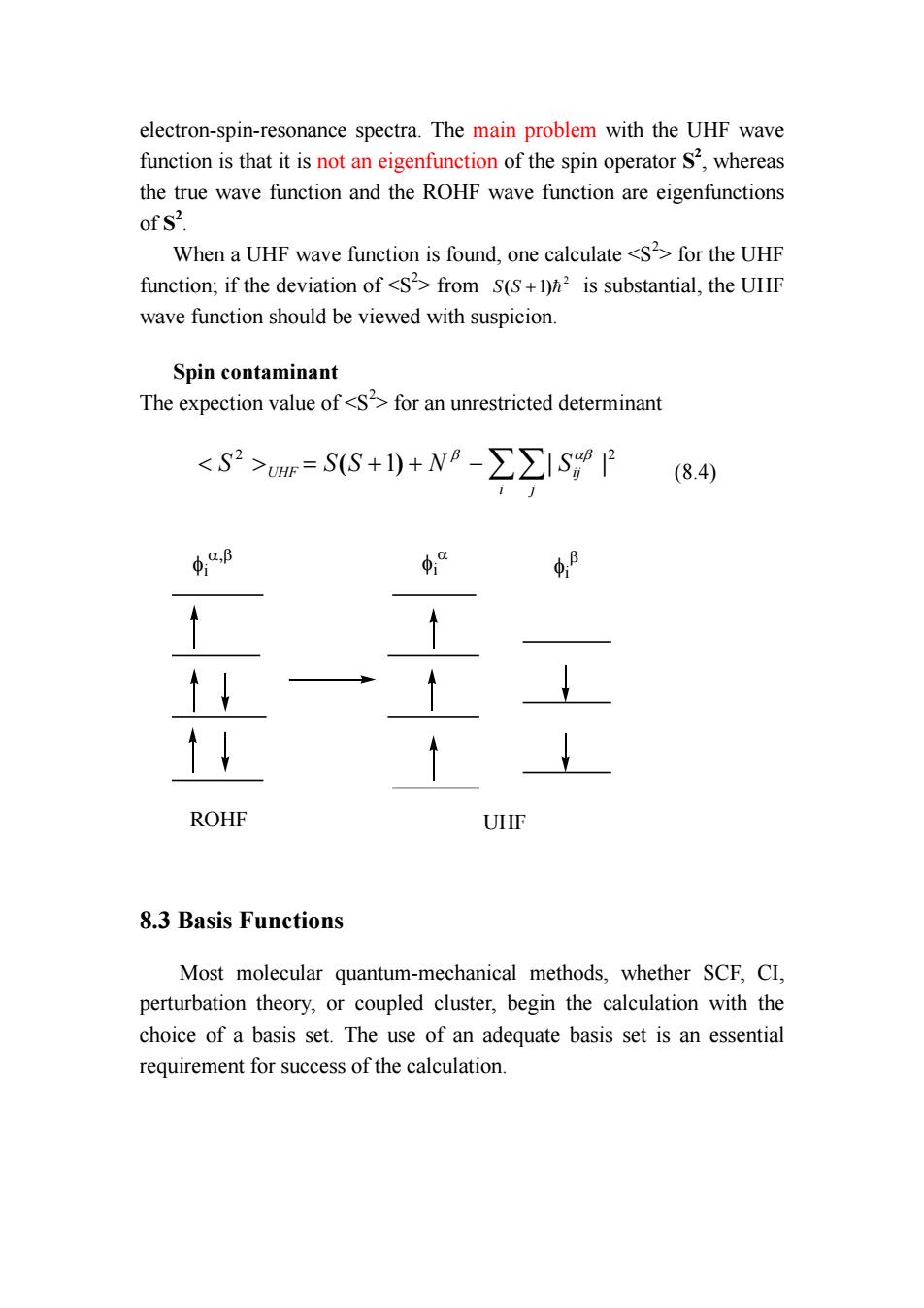
electron-spin-resonance spectra.The main problem with the UHF wave function is that it is not an eigenfunction of the spin operator S2,whereas the true wave function and the ROHF wave function are eigenfunctions of S2. When a UHF wave function is found,one calculate for the UHF function;if the deviation of from S(S+)h is substantial,the UHF wave function should be viewed with suspicion Spin contaminant The expection value of=SS+1)+N8-∑∑ISP84 4.B φ“ ↑↓ ROHF UHF 8.3 Basis Functions Most molecular quantum-mechanical methods,whether SCF,CI, perturbation theory,or coupled cluster,begin the calculation with the choice of a basis set.The use of an adequate basis set is an essential requirement for success of the calculation
electron-spin-resonance spectra. The main problem with the UHF wave function is that it is not an eigenfunction of the spin operator S2 , whereas the true wave function and the ROHF wave function are eigenfunctions of S2 . When a UHF wave function is found, one calculate for the UHF function; if the deviation of from 2 S(S +1)= is substantial, the UHF wave function should be viewed with suspicion. Spin contaminant The expection value of for an unrestricted determinant 2 2 = ( +1) + −∑∑| | i j S UHF S S N Sij β αβ (8.4) ROHF UHF φi α,β φi α φi β 8.3 Basis Functions Most molecular quantum-mechanical methods, whether SCF, CI, perturbation theory, or coupled cluster, begin the calculation with the choice of a basis set. The use of an adequate basis set is an essential requirement for success of the calculation

Slater orbitals(STOs) A Slater function centered on nucleus a has the form X=Nra-eYm(0) (8.5) Molecular orbital (MO) 4=∑cn (8.6) where the 's are the STO basis functions.We have LC-STO MOs. For polyatomic molecules,the LC-STO method uses STOs centered on each of the atoms.The presence of more than two atoms causes difficulties in evaluating the needed integrals Gaussian-type orbitals(GTOs) Computer evaluation of three-and four-center integrals over STO basis functions is very time consuming.To simplify molecular integral evaluation,Boys proposed in 1950 the use of Gaussian-type functions (GTOs)instead of STOs for the atomic orbitals in an LCAO wave function. A Cartesian Gaussian centered on nucleus a is defined as 8i=x。yze (8.7) where N is the normalization constant,i,j,and k are nonnegative integers, and a is positive orbital exponent. i+j+k=0,s-type Gaussian itj+k=1,p-type Gaussians,px pP: i+j+k=2,d-type Gaussians,6 d-type Gaussians, with the factors x2,y,z,xy,xz,yz.They can form five linear combinations having the same angular behavior as the five 3d AOs;the sixth combination with the factor x2+y2+z2=r2 is like a 3s AO
Slater orbitals (STOs) A Slater function centered on nucleus a has the form ( , ) a a m l n r r Nra e Y a χ θ φ − −ξ = 1 (8.5) Molecular orbital (MO) r r i ri φ = ∑ c χ (8.6) where the χr’s are the STO basis functions. We have LC-STO MOs. For polyatomic molecules, the LC-STO method uses STOs centered on each of the atoms. The presence of more than two atoms causes difficulties in evaluating the needed integrals. Gaussian-type orbitals (GTOs) Computer evaluation of three- and four-center integrals over STO basis functions is very time consuming. To simplify molecular integral evaluation, Boys proposed in 1950 the use of Gaussian-type functions (GTOs) instead of STOs for the atomic orbitals in an LCAO wave function. A Cartesian Gaussian centered on nucleus a is defined as 2 a k r a j a i ijk a g Nx y z e −α = (8.7) where N is the normalization constant, i, j, and k are nonnegative integers, and α is positive orbital exponent. i+j+k = 0, s-type Gaussian i+j+k = 1, p-type Gaussians, px, py, pz. i+j+k = 2, d-type Gaussians, 6 d-type Gaussians, with the factors x2 , y 2 , z 2 , xy, xz, yz. They can form five linear combinations having the same angular behavior as the five 3d AOs; the sixth combination with the factor x 2 + y2 + z2 = r 2 is like a 3s AO
Spherical Gaussians Nr-eaG(y,m±ym)/W2 (8.8) LC-GTOAO A Gaussian function does not have the desired cusp at the nucleus and hence gives a poor representation of an AO for small values ofr To get an accurate representation of an AO,we must use a linear combination of several Gaussians-LC-GTO AOs.Therefore,an LC-GTO SCF MO calculation involves evaluation of very many more integrals than the corresponding LC-STO SCF MO calulation. Gaussian integral evaluation takes much less computer time than Slater integral evaluation.This is because the product of two Gaussian centered at two different points is equal to a single Gaussian centered at a third point.Thus all three-and four-center two-electron repulsion integrals are reduced to two-center integrals. The STO Basis Set Terminology Minimal (or minimum)basis set:One STO for each inner-shell and valence-shell AO of each atom.For example,for C2H2 a minimal basis set consists of 1s,2s,2p 2py,and 2p AOs on each carbon and Is STO on each hydrogen,there are five STOs on each C and one on each H,for a total of twelve basis functions,such a set is denoted by(2slp)for the carbon functions and (1s)for the hydrogen functions,a notation which is further abbreviated to(2s1p/1s). The numbers of basis functions in a minimal set for the first part of the periodic table are H,He Li-Ne Na-Ar K,Ca Sc-Kr 115 91318 A Double-Zeta (DZ)basis set:Replacing each STO of a minimal basis set by two STOs that differ in their orbital exponents(zeta). For example,for C2H2 a double-zeta basis set consists of (1s,Is'), (2s,2s),(2px,2px),(2py,2p),and (2p22p2)AOs on each carbon and
Spherical Gaussians 2 1 * ( )/ 2 a n mm r Nr e Y Y a ll − −α ± (8.8) LC-GTO AO A Gaussian function does not have the desired cusp at the nucleus and hence gives a poor representation of an AO for small values of ra. To get an accurate representation of an AO, we must use a linear combination of several Gaussians — LC-GTO AOs. Therefore, an LC-GTO SCF MO calculation involves evaluation of very many more integrals than the corresponding LC-STO SCF MO calulation. Gaussian integral evaluation takes much less computer time than Slater integral evaluation. This is because the product of two Gaussian centered at two different points is equal to a single Gaussian centered at a third point. Thus all three- and four-center two-electron repulsion integrals are reduced to two-center integrals. The STO Basis Set Terminology Minimal (or minimum) basis set: One STO for each inner-shell and valence-shell AO of each atom. For example, for C2H2 a minimal basis set consists of 1s, 2s, 2px, 2py, and 2pz AOs on each carbon and 1s STO on each hydrogen; there are five STOs on each C and one on each H, for a total of twelve basis functions; such a set is denoted by (2s1p) for the carbon functions and (1s) for the hydrogen functions, a notation which is further abbreviated to (2s1p/1s). The numbers of basis functions in a minimal set for the first part of the periodic table are H, He Li-Ne Na-Ar K, Ca Sc-Kr 1 5 9 13 18 A Double-Zeta (DZ) basis set: Replacing each STO of a minimal basis set by two STOs that differ in their orbital exponents ζ (zeta). For example, for C2H2 a double-zeta basis set consists of (1s,1s’), (2s,2s’), (2px,2px’), (2py,2py’), and (2pz,2pz’) AOs on each carbon and

(1s,Is')STOs on each hydrogen,for a total of twenty-four basis functions;this is(4s2p/2s)basis set A Split-valence (SV)basis set:Minimal for the inner-shell and double zeta for the valence AOs Polarization functions A set of three 2p functions(2px,2py,2pz)on each hydrogen atom A set of five3 d functions on each“first--row'and“second-row”atom. A set of seven 4f functions on each"third-row"atom. A double-zeta plus polarization:DZ+P For improved accuracy,higher-I polarization functions can be added. Contracted Gaussian-type functions Instead of using the individual Gaussian functions (8.7)as basis functions,the current practice is to take basis function as a linear combination of a small of Gaussians,according to X,=∑dn8 (8.9) where the gu's are Cartesian Gaussians [Eq.(8.7)]centered on the same atom having the same i,j,k values as one another,but different a's.The coefficients dr are constants that are held fixed during the calculation In (15.12),Xr called a contracted Gaussian-type function (CGTF)and the gu's are called primitive Gaussians. By using contracted Gaussians instead of primitive Gaussians as basis set,the number of variational coefficients to be determined is reduced, which gives large savings in computational time with little loss in accuracy if the contracted coefficients d are well chosen. Several methods exist to form contracted Gaussian sets.Minimal CGTF sets are usually formed by fitting STOs.Each STO is approximated as a linear combination of N Gaussian functions(called STO-NG basis set). where the coefficients in the linear combination and the Gaussian orbital exponents are chosen to give the best least-square-fit to the STO
(1s,1s’) STOs on each hydrogen, for a total of twenty-four basis functions; this is (4s2p/2s) basis set. A Split-valence (SV) basis set: Minimal for the inner-shell and double zeta for the valence AOs. Polarization functions: A set of three 2p functions (2px, 2py, 2pz) on each hydrogen atom. A set of five 3d functions on each “first-row” and “second-row” atom. A set of seven 4f functions on each “third-row” atom. …… A double-zeta plus polarization: DZ+P For improved accuracy, higher-l polarization functions can be added. Contracted Gaussian-type functions Instead of using the individual Gaussian functions (8.7) as basis functions, the current practice is to take basis function as a linear combination of a small of Gaussians, according to u u χ r = ∑dur g (8.9) where the gu’s are Cartesian Gaussians [Eq.(8.7)] centered on the same atom having the same i, j, k values as one another, but different α’s. The coefficients dur are constants that are held fixed during the calculation. In (15.12), χr called a contracted Gaussian-type function (CGTF) and the gu’s are called primitive Gaussians. By using contracted Gaussians instead of primitive Gaussians as basis set, the number of variational coefficients to be determined is reduced, which gives large savings in computational time with little loss in accuracy if the contracted coefficients dur are well chosen. Several methods exist to form contracted Gaussian sets. Minimal CGTF sets are usually formed by fitting STOs. Each STO is approximated as a linear combination of N Gaussian functions (called STO-NG basis set), where the coefficients in the linear combination and the Gaussian orbital exponents are chosen to give the best least-square-fit to the STO,

Another way to form contracted Gaussians is to start with atomic CTF SCF calculations.Huzinaga used a (9s5p)basis set of uncontracted Gaussians to do SCF calculations on the atoms Li-Ne.For example,for the ground state of the O atom,the optimized orbital exponents of the nine s-type basis GTFs and the expansion coefficients were found to be Exponents 1s Coefficients 2s Coefficients g17817 0.0012 -0.0003 g21176 0.009 -0.002 g3273.2 0.043 -0.010 g481.2 0.144 -0.036 gs27.2 0.356 -0.095 g69.53 0.461 -0.196 873.41 0.140 -0.037 880.94 -0.0006 0.596 g90.285 0.001 0.526 Suppose we want to form a split-valence [3s2p]set of contracted GTFs for O.We can take following contracted scheme 1s=N(0.0012g1+0.009g2+0.043g3+0.144g4+0.356g5+0.461g6 +0.14g7) 2s=N(-0.196g6+0.596gs) 2s'=g9 Standard basis sets:(by Pople and coworkers) ST0-3G3-21G4-31G6-31G6-311G. 3-21G*,6-31G*,… In the 3-21G set,each inner-shell AO(1s for Li-Ne;1s,2s,2p for Na-Ar; and so on)is represented by a single CGTF that is a linear combination of three primitive Gaussian;for each valence-shell AO(1s for H;2s and the 2p's for Li-Ne;..),there are two basis functions,one of which is a CGTF that is a linear combination of two Gaussian primitives and one
Another way to form contracted Gaussians is to start with atomic CTF SCF calculations. Huzinaga used a (9s5p) basis set of uncontracted Gaussians to do SCF calculations on the atoms Li-Ne. For example, for the ground state of the O atom, the optimized orbital exponents of the nine s-type basis GTFs and the expansion coefficients were found to be Exponents 1s Coefficients 2s Coefficients g1 7817 0.0012 -0.0003 g2 1176 0.009 -0.002 g3 273.2 0.043 -0.010 g4 81.2 0.144 -0.036 g5 27.2 0.356 -0.095 g6 9.53 0.461 -0.196 g7 3.41 0.140 -0.037 g8 0.94 -0.0006 0.596 g9 0.285 0.001 0.526 Suppose we want to form a split-valence [3s2p] set of contracted GTFs for O. We can take following contracted scheme 1s = N(0.0012g1 + 0.009g2 + 0.043g3 + 0.144g4 +0.356g5 + 0.461g6 +0.14g7) 2s = N’(-0.196g6 + 0.596 g8) 2s’ = g9 …… Standard basis sets: (by Pople and coworkers) STO-3G, 3-21G, 4-31G, 6-31G, 6-311G,… 3-21G*, 6-31G*, … In the 3-21G set, each inner-shell AO (1s for Li-Ne; 1s, 2s, 2p for Na-Ar; and so on) is represented by a single CGTF that is a linear combination of three primitive Gaussian; for each valence-shell AO (1s for H; 2s and the 2p’s for Li-Ne; …), there are two basis functions, one of which is a CGTF that is a linear combination of two Gaussian primitives and one
which is a single diffuse Gaussian. ECPbasis sets Such basis sets are available in the widely used ab initio programs GAUSSIAN 98,GAMESS,HONDO,etc. 8.4 The SCF MO Treatment of H2O A minimal-basis-set MO treatment Basis functions:O1s,02s,O2px,O2py,O2p;HiIs,H21s. Linear combinations of these 7 basis AOs give LCAO approximations to the seven lowest MOs of water. Symmetry-adapted basis functions. E C2(z)G(xz)(yz) A 1 1 1 1 A 1 1 1 -1 B) 1 -1 1 -1 B2 1 -1 -1 1 Each oxygen AO transforms according to one of the symmetry species of water and can serve as a symmetry orbital.However,neither of the two hydrogen 1s AOs belongs to a symmetry species of water,and we must construct two symmetry orbitals from these AOs.Consider the following linear combinations Hils+H2ls (8.10) Hils-H21s (8.11) Examination of the effects of the other three symmetry operators shows (8.10)and (8.11)to belong to the symmetry species a and b2
which is a single diffuse Gaussian. ECP basis sets Such basis sets are available in the widely used ab initio programs GAUSSIAN 98, GAMESS, HONDO, etc. 8.4 The SCF MO Treatment of H2O A minimal-basis-set MO treatment Basis functions: O1s, O2s, O2px, O2py, O2pz; H11s, H21s. Linear combinations of these 7 basis AOs give LCAO approximations to the seven lowest MOs of water. Symmetry-adapted basis functions. H1 H2 O y z E C2(z) σv(xz) σv(yz) A1 A2 B1 B2 1 1 1 1 1 1 1 1 -1 -1 -1 1 -1 -1 -1 1 Each oxygen AO transforms according to one of the symmetry species of water and can serve as a symmetry orbital. However, neither of the two hydrogen 1s AOs belongs to a symmetry species of water, and we must construct two symmetry orbitals from these AOs. Consider the following linear combinations: H11s + H21s (8.10) H11s - H21s (8.11) Examination of the effects of the other three symmetry operators shows (8.10) and (8.11) to belong to the symmetry species a1 and b2