Chapter 5 The structure of polyatomic molecules (A)
Chapter 5 The structure of polyatomic molecules (A)

5.1 Theory of hybrid orbital and atomic orbital hybridization 1.Hybrid orbital theory---Proposed by Pauling in 1928 Chemical bonding theories MOLECULAR ORBITAL THEORY Valence electrons are delocalized Valence electrons spread over entire molecule VALENCE BOND THEORY Valence electrons are localized between atoms (or are lone pairs). Half-filled atomic orbitals overlap to form bonds
MOLECULAR ORBITAL THEORY • Valence electrons are delocalized • Valence electrons spread over entire molecule. Chemical bonding theories VALENCE BOND THEORY • Valence electrons are localized between atoms (or are lone pairs). • Half-filled atomic orbitals overlap to form bonds. §5.1 Theory of hybrid orbital and atomic orbital hybridization 1. Hybrid orbital theory ---Proposed by Pauling in 1928

Simplified MO diagram of diatomic molecules Og(2S)=(44+42B) n9 0g(2p)=(4p.A-4p,B) LUMO Og(2sp)= 20 9 C(p2s4+p2sB)±C2(02p.A-02p.B) HOMO (C024±C292p,4)+(C102sB干C292p.B) sp-hybridization 3 ,(2sp)= (C924±C202p4)-(C102,B干C92p,B) N2:KK(1oe)21o)(1π)(2o) The narrow energy gap between 2s and 2pz orbitals in a N atom also enables the interatomic interaction between 2s(N1)and 2pz(N2)!
The narrow energy gap between 2s and 2pz orbitals in a N atom also enables the interatomic interaction between 2s (N1) and 2pz(N2)!
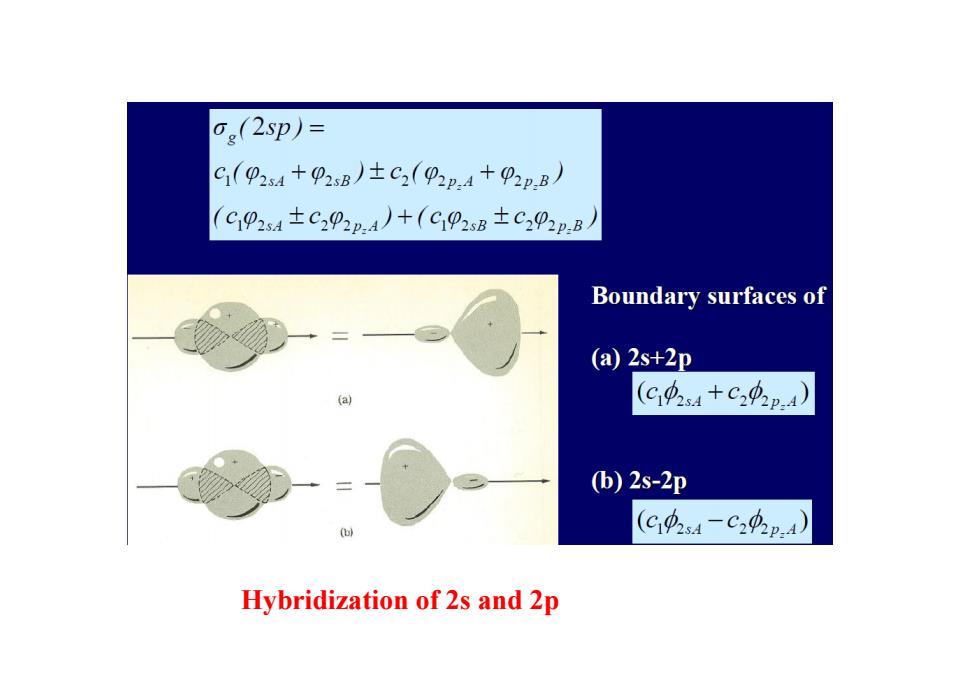
6e(2p)= C1(92s4+P2sB)±C2(p2:4+P2p.B) (C102s4±C22p,A)+(C92sB±C202p,B Boundary surfaces of (a)2s+2p (C4s4+C20p.4) (b)2s-2p (C04-C24p.4) Hybridization of 2s and 2p
Hybridization of 2s and 2p

Central Themes of Valence Bond Theory Basic Principle of Valence Bond Theory:a covalent bond forms when the orbitals from two atoms overlap and a pair of electrons occupies the region between the nuclei. 1)Opposing spins of the electron pair. 2)Maximum overlap of bonding orbitals. 3)Hybridization of atomic orbitals. Pauling proposed that the valence atomic orbitals in the molecule are different from those in the isolated atoms.We call this Hybridization!
Central Themes of Valence Bond Theory 1) Opposing spins of the electron pair. 2) Maximum overlap of bonding orbitals. 3) Hybridization of atomic orbitals. Pauling proposed that the valence atomic orbitals in the molecule are different from those in the isolated atoms. We call this Hybridization! Basic Principle of Valence Bond Theory: a covalent bond forms when the orbitals from two atoms overlap and a pair of electrons occupies the region between the nuclei
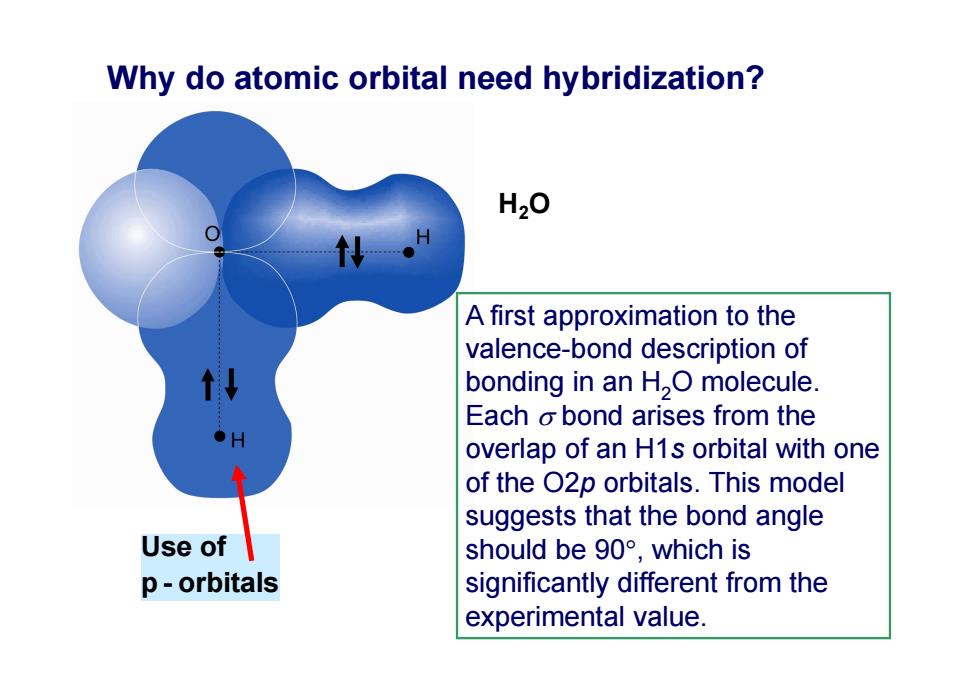
Why do atomic orbital need hybridization? H20 A first approximation to the valence-bond description of bonding in an H,O molecule. Each o bond arises from the overlap of an H1s orbital with one of the O2p orbitals.This model suggests that the bond angle Use of should be90°,which is p-orbitals significantly different from the experimental value
Use of p - orbitals H2O Why do atomic orbital need hybridization? A first approximation to the valence-bond description of bonding in an H2O molecule. Each bond arises from the overlap of an H1s orbital with one of the O2p orbitals. This model suggests that the bond angle should be 90, which is significantly different from the experimental value

104.5 b1 b2 a H20 H:03 The admixture of 2s 2p orbitals H is required. Inequivalent sp3 hybridization of O(2sp)AOs
Px Py a b 104.5 b1 b2 H O H H2O The admixture of 2s & 2p orbitals is required. Inequivalent sp3 hybridization of O(2sp) AOs
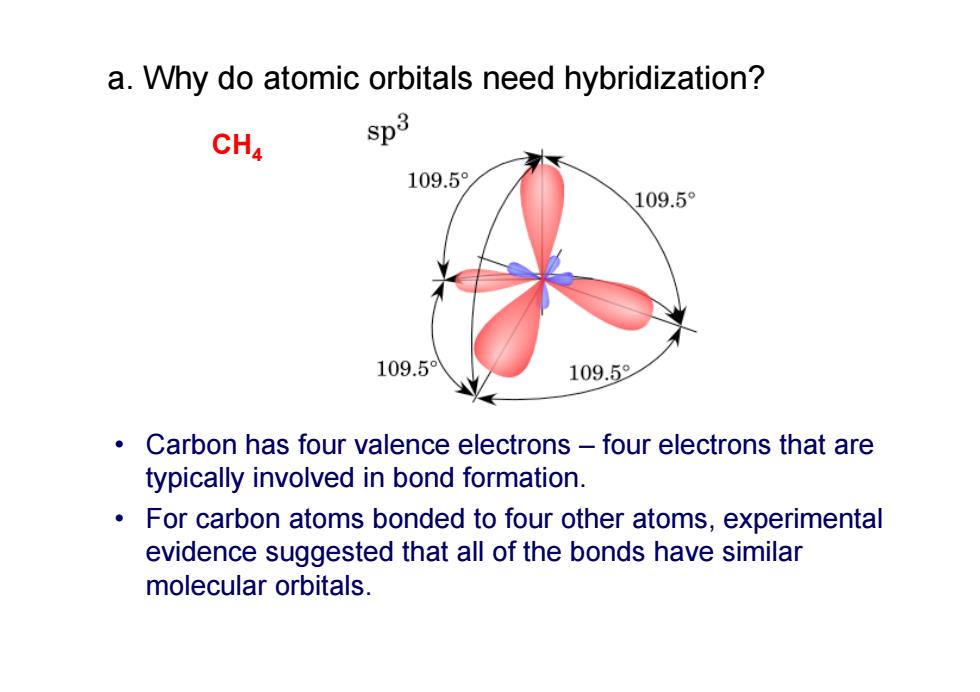
a.Why do atomic orbitals need hybridization? CH4 $p3 109.5 109.5° 109.5 109.5 Carbon has four valence electrons-four electrons that are typically involved in bond formation. For carbon atoms bonded to four other atoms,experimental evidence suggested that all of the bonds have similar molecular orbitals
• Carbon has four valence electrons – four electrons that are typically involved in bond formation. • For carbon atoms bonded to four other atoms, experimental evidence suggested that all of the bonds have similar molecular orbitals. CH 4 a. Why do atomic orbitals need hybridization?

b.How do atomic orbitals hybridize? 4。=C2,+C202m+C3py+C44p ④k=∑c4 i=i∑cx=∑ic4=∑cxi0 =∑cxE,4,=E:∑cx中 If we begin with n AO's,we must end up with n orbitals after hybridization. All n hybrids are equivalent except for directionality same energy
b. How do atomic orbitals hybridize? i i i k ik i ik i i i i ik i i ik i k ik i i k ik c E E c H H c Hc c H c ˆ ˆ ˆ ˆ h s px py pz c c c c 1 2 22 3 2 42 If we begin with n AO’s, we must end up with n orbitals after hybridization. All n hybrids are equivalent except for directionality same energy

2.Construction of hybrid orbitals
2. Construction of hybrid orbitals