Chapter 6 Polyatomic molecules (II) 6.1 Electron-deficient multi-center bonds and electron-deficient molecules 6.1.1 Boranes and their relatives
Chapter 6 Polyatomic molecules (II) 6.1 Electron-deficient multi-center bonds and electron-deficient molecules 6.1.1 Boranes and their relatives
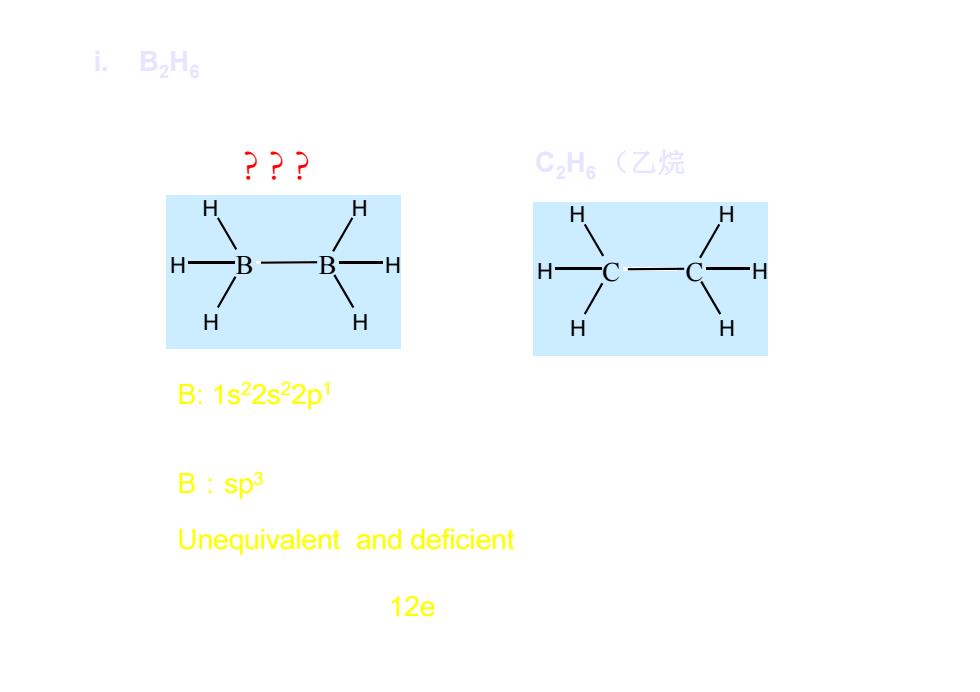
i.B2He ??? CH。(乙烷 B:1s22s22p B:sp3 Unequivalent and deficient 12e
? ? ? B H H H B H H H i. B2H6 C H H H C H H H C2H6 (乙烷 ethane) (乙硼烷 diborane; boroethane ) B: 1s22s22p1 C: 1s22s22p2 Hybridization orbitals B:sp3 C: sp3 Unequivalent and deficient equivalent orbitals valence electron:12e 14e
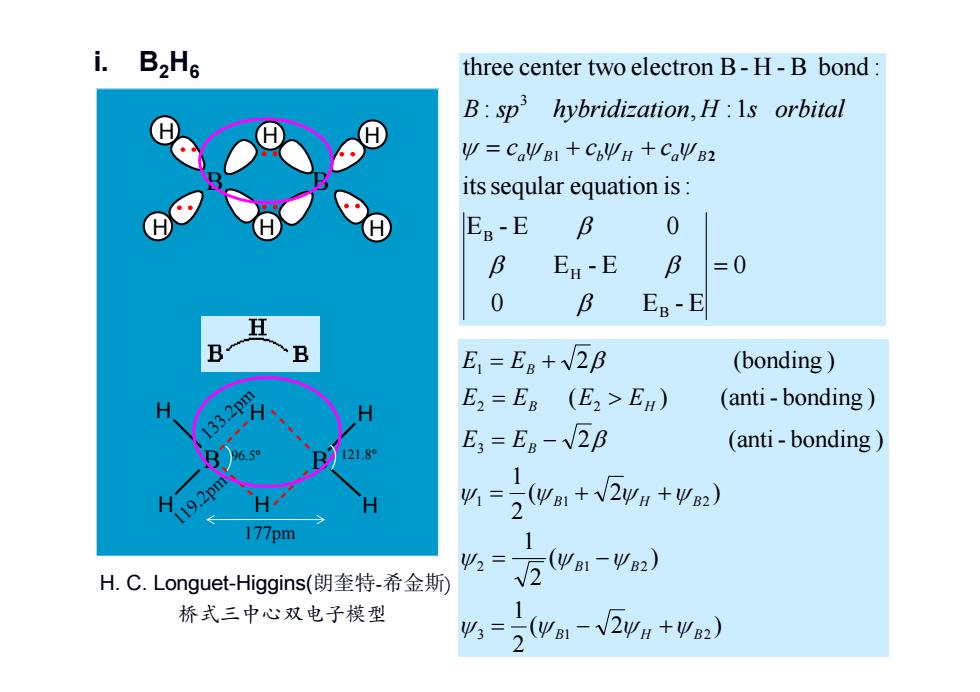
i.B2He three center two electron B-H-B bond: B:sp hybridization,H:Is orbital y=CaΨB1+CbΨH+CaΨB2 its seqular equation is: H E8-E B 0 B En-E B =0 0 B E8-E B B E=Eg+2B (bonding) E2=Eg (E2>En) (anti-bonding) 33 E;=E8-2B (anti-bonding) 12189 1 19.2pm 必1= (ya1+V2yH+y2) 2 177pm 1 Ψ2= 5(ΨB1-ΨB2) H.C.Longuet-Higgins(朗奎特-希金斯 桥式三中心双电子模型 1 =2 (9B1-V2yH+Ψ2)
p125 HMO method 0 0 E - E E - E E - E 0 itsseqular equation is: : , :1 three center two electron B- H - B bond : B H B 1 3 a B b H a B2 c c c B sp hybridization H s orbital i. B2H6 ( 2 ) 21 ( ) 21 ( 2 ) 21 2 (anti - bonding ) ( ) (anti - bonding ) 2 (bonding ) 3 1 2 2 1 2 1 1 2 3 2 2 1 B H B B B B H B B B H B E E E E E E E E B H H H H B H H 96.5° 121.8° B H H H H H H B H. C. Longuet-Higgins(朗奎特-希金斯) 177pm 桥式三中心双电子模型
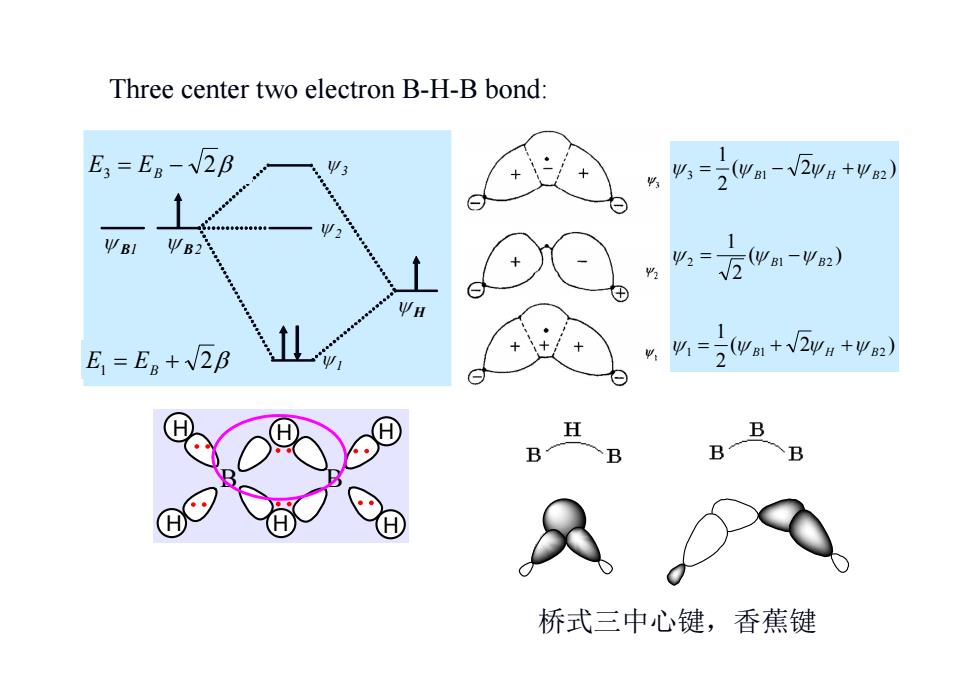
Three center two electron B-H-B bond: E3 E8-2B 1 Ψ3 %=2wa-V2y4+42) Ψ ΨB1 ΨB2 1 业2= (Ψ1-Ψ82) √2 E=Eg+2B %=2wam+V2y+2) H B B 桥式三中心键,香蕉键
B H H H H H H B Three center two electron B-H-B bond: ( 2 ) 21 ( ) 21 ( 2 ) 21 1 1 2 2 1 2 3 1 2 B H B B B B H B B1 B2 H 1 2 3 E1 EB 2 E3 EB 2 桥式三中心键,香蕉键
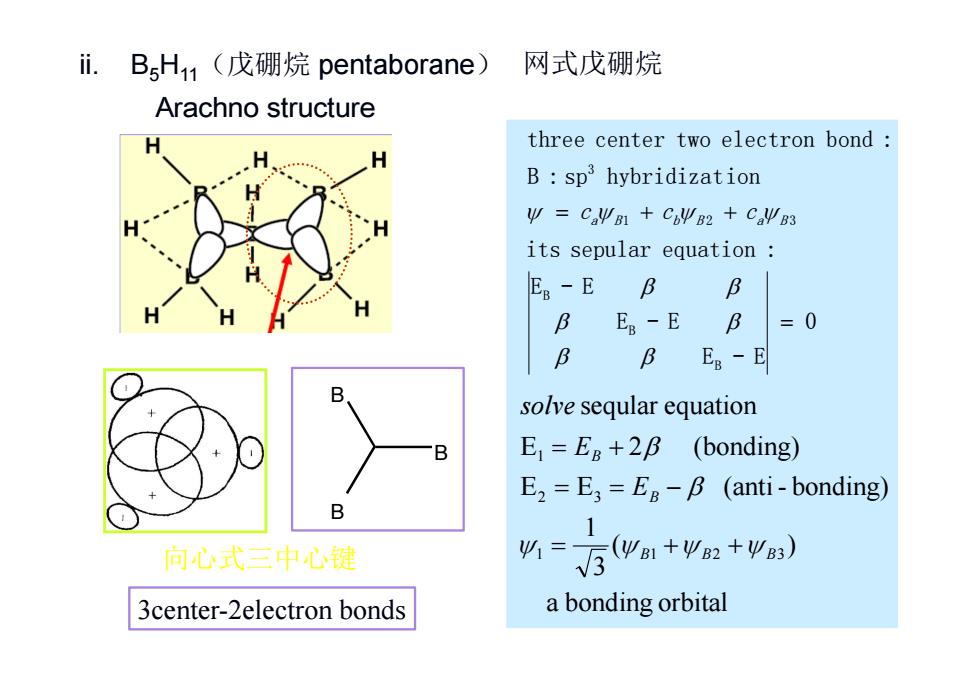
i.BsH1(戊硼烷pentaborane) 网式戊硼烷 Arachno structure three center two electron bond B:sp3hybridization Ψ=CaΨ1+CΨB2+CΨB3 its sepular equation ER-E 6 B B Es-E B =0 B B Ep-E solve seqular equation B E=Eg+28 (bonding) E2 =E3=E8-B (anti-bonding) B 1 向心式中心键 %=后wm+w:+wa) 3center-2electron bonds a bonding orbital
ii. B5H11(戊硼烷 pentaborane) 0 E - E E - E E - E its sepular equation : B : sp hybridization three center two electron bond : B B B 1 2 3 3 ca B cb B ca B a bonding orbital ( ) 31 E E (anti - bonding) E 2 (bonding) seqular equation 1 1 2 3 2 3 1 B B B B B E E solve B B B 3center-2electron bonds 向心式三中心键 Arachno structure 网式戊硼烷
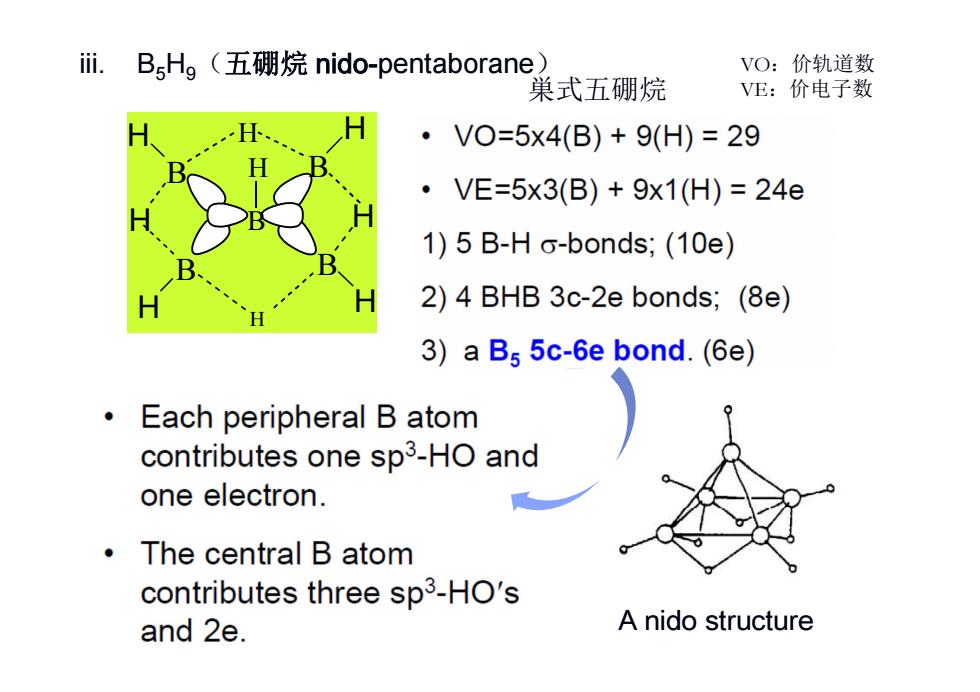
ii. B,Hg(五硼烷nido-pentaborane VO:价轨道数 巢式五硼烷 VE:价电子数 ·VO=5x4(B)+9(H)=29 ·VE=5x3(B)+9x1(H)=24e 1)5 B-H o-bonds;(10e) 2)4 BHB 3c-2e bonds;(8e) 3)a B5 5c-6e bond.(6e) 0 Each peripheral B atom contributes one sp3-HO and one electron. ·The central B atom contributes three sp3-HO's and 2e. A nido structure
iii. B5H9(五硼烷 nido-pentaborane) B H H B H H B H B H B H H H A nido structure VO:价轨道数 巣式五硼烷 VE:价电子数
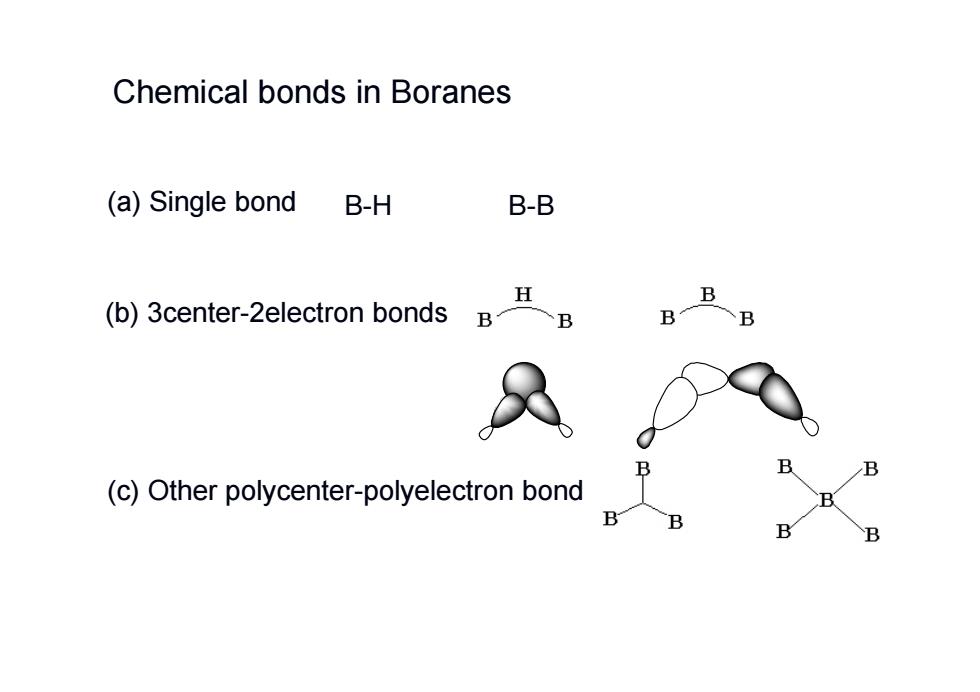
Chemical bonds in Boranes (a)Single bond B-H B-B H (b)3center-2electron bonds B B (c)Other polycenter-polyelectron bond B
B-H B-B (c) Other polycenter-polyelectron bond (b) 3center-2electron bonds (a) Single bond Chemical bonds in Boranes
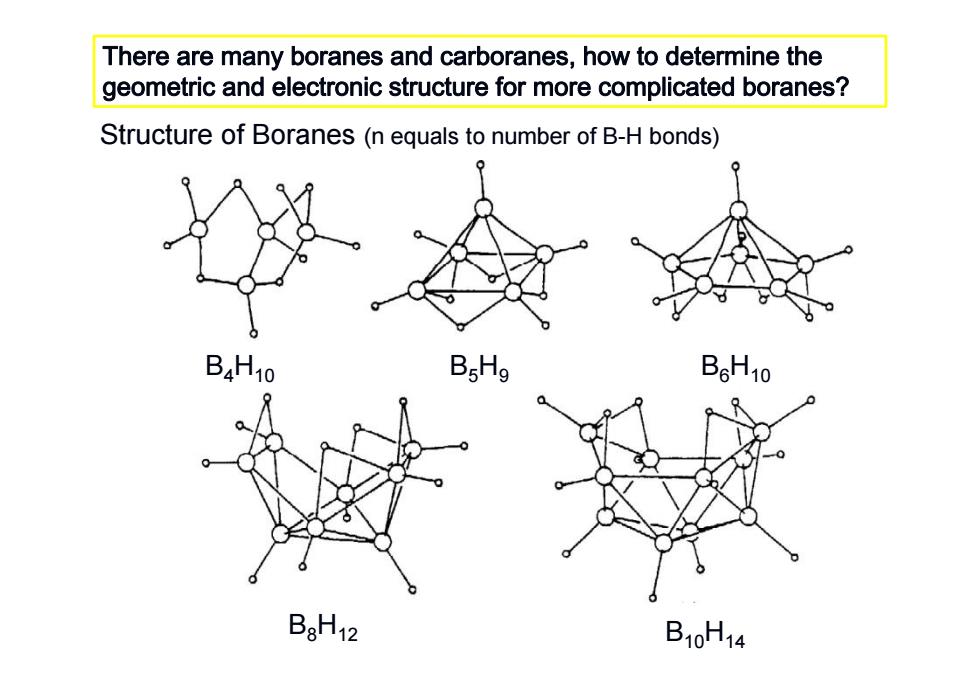
There are many boranes and carboranes,how to determine the geometric and electronic structure for more complicated boranes? Structure of Boranes(n equals to number of B-H bonds) B4H10 B.Hg B6H10 BaH12 B10H14
There are many boranes and carboranes, how to determine the geometric and electronic structure for more complicated boranes ? Structure of Boranes (n equals to number of B-H bonds) B 4 H10 B 5 H 9 B 6 H10 B 8 H12 B10 H14

6.1.2 For B H+m's open structure(including nido and arachno) In the mid 1950s,Lipscomb proposed the styx method to predict the topological structures of boranes BHn+m with open structures.Lipscomb was awarded Nobel prize "for his studies on the structure of boranes"in 1976.he passed away on Apr. 14,2011. William Lipscomb(American) Nobel speech: Boranes and their chemical bonding uilliam
6.1.2 For BnHn+m’s open structure (including nido and arachno) In the mid 1950s, Lipscomb proposed the styx method to predict the topological structures of boranes BnHn+m with open structures. Lipscomb was awarded Nobel prize “for his studies on the structure of boranes” in 1976. he passed away on Apr. 14, 2011. William Lipscomb(American) Nobel speech: Boranes and their chemical bonding

The topological analysis for BHn+m's with open structure n:the number of B atoms,also the minimal number of B-H bonds m:the number of extra H atomes 硼烷结构的拓扑分析法styx method B:Ho B
B H H B H H B H B H B H H H B B H B B B B B H B B B B H H B B s t y x B5H9 硼烷结构的拓扑分析法 styx method The topological analysis for BnHn+m’s with open structure n: the number of B atoms, also the minimal number of B-H bonds m:the number of extra H atomes