
Structural Chemistry 结构化学是研究原子、分子和晶体的微观结构,研究原子和分 子运动规律,研究物质的结和性能关系的科学 主要研究从原子、分子片、分子、超分子,到分子和原子的各种 不同尺度和不同复杂程度的聚集态和组装态的合成和反应,分离 和分析,结构和形态,物理性能和生物活性及其规律和应用的自 然科学 --- 徐光宪 1998年诺贝尔化学奖获得者Kohn和Pople认为: “量子化学已经发展成为广大化学家所使用的工具,将化学带入 一个新时代,在这个新时代里实验和理论能够共同协力探讨分子 体系的性质。化学不再是纯粹的实验科学了
Structural Chemistry 主要研究从原子、分子片、分子、超分子,到分子和原子的各种 不同尺度和不同复杂程度的聚集态和组装态的合成和反应,分离 和分析,结构和形态,物理性能和生物活性及其规律和应用的自 然科学 –-- 徐光宪 1998年诺贝尔化学奖获得者Kohn和Pople认为: “量子化学已经发展成为广大化学家所使用的工具,将化学带入 一个新时代,在这个新时代里实验和理论能够共同协力探讨分子 体系的性质。化学不再是纯粹的实验科学了” 结构化学是研究原子、分子和晶体的微观结构,研究原子和分 子运动规律,研究物质的结和性能关系的科学

The Nobel Prize in Chemistry 2013 Nobel Prize in Chemistry 1981 Martin Karplus,Michael Levitt,Arieh Warshel Fukui Hoffmann The Nobel Prize in Chemistry 2013 ©Nobel Media AB Photo:Kellana via Martin Karplus Wikimedia Commons Michael Levitt Arieh Warshel QM/MM boundary The Nobel Prize in Chemistry 2013 was awarded jointly to Martin Karplus, Solution Michael Levitt and Arieh Warshel "for the development of multiscale models for complex chemical systems". 2013年诺贝尔化学奖获奖理由:复杂体系多尺度模型
2013年诺贝尔化学奖获奖理由:复杂体系多尺度模型 Nobel Prize in Chemistry 1981 Fukui & Hoffmann

Know-How about Structural Chemistry ◆3+2+1原则 3种理论:量子理论,化学键理论,点阵理论 3种结构:原子结构,分子结构,晶体结构 3个基础:量子化学基础,对称性原理基础,结晶化学基础 2个因素:电子因素,空间因素 1条主线:结构决定性能,性能反映结构 理解为主,记忆为辅(预习-复习--总结) ◆发展的观点 分子→超分子,微观→介观(纳米)→宏观,相对论? 光速不变?
Know-How about Structural Chemistry 3+2+1原则 3种理论:量子理论,化学键理论,点阵理论 3种结构:原子结构,分子结构,晶体结构 3个基础:量子化学基础,对称性原理基础,结晶化学基础 2个因素:电子因素,空间因素 1条主线:结构决定性能,性能反映结构 理解为主,记忆为辅(预习--- 复习--- 总结 ) 发展的观点 分子 →超分子,微观 →介观(纳米) →宏观,相对论? 光速不变? …
What is Chemistry The branch of natural science that deals with composition,structure, properties of substances and the changes they undergo. Chemistry Chem is try
What is Chemistry The branch of natural science that deals with composition, structure, properties of substances and the changes they undergo. Chemistry = Chem + is + try ?
Structure vs.Properties The structure determines properties Properties reflect the structure
The structure determines properties Properties reflect the structure Structure vs. Properties
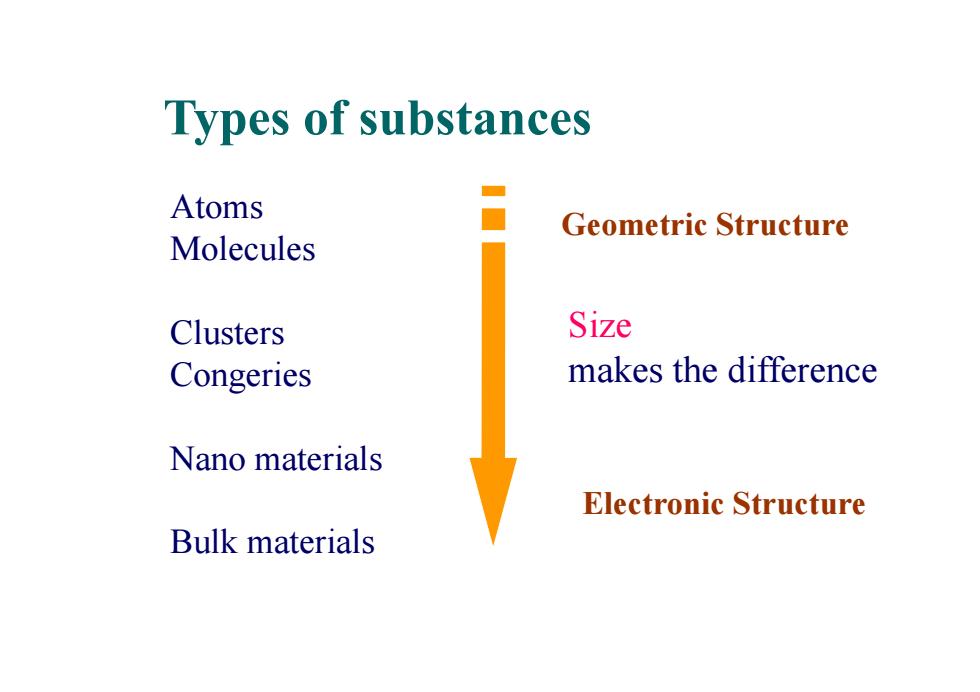
Types of substances Atoms Geometric Structure Molecules Clusters Size Congeries makes the difference Nano materials Electronic Structure Bulk materials
Types of substances Atoms Molecules Clusters Congeries Nano materials Bulk materials Geometric Structure Size makes the difference Electronic Structure
Basic Units Atom The basic building block of all matter.The smallest particle of an element that has the same properties as the element. Composed of an electron cloud and a central nucleus
Atom The basic building block of all matter. The smallest particle of an element that has the same properties as the element. Composed of an electron cloud and a central nucleus. Basic Units
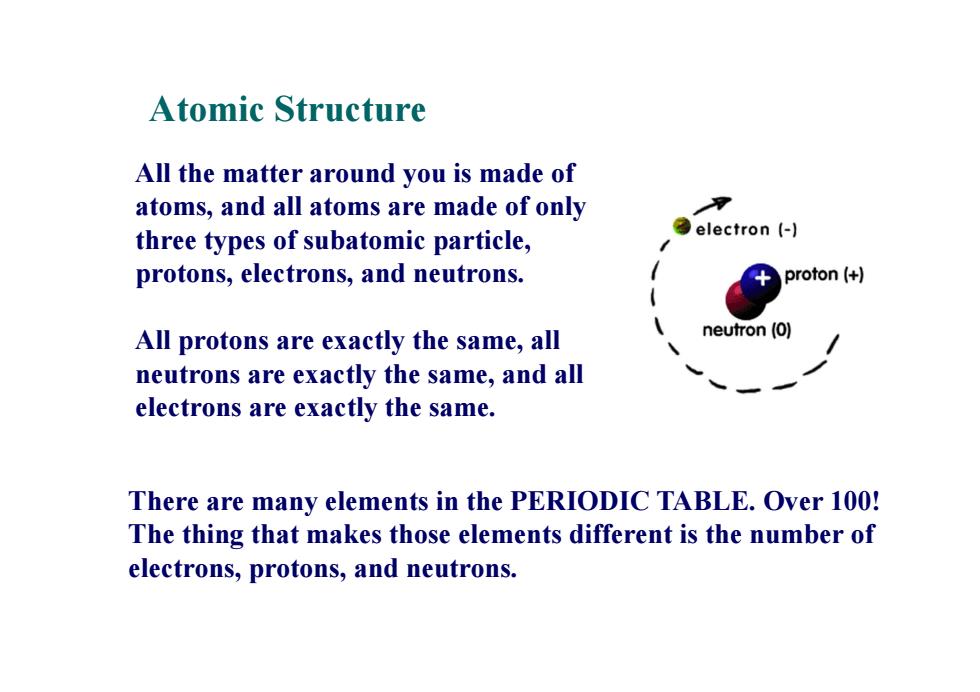
Atomic Structure All the matter around you is made of atoms,and all atoms are made of only three types of subatomic particle, ②electron(-l protons,electrons,and neutrons. proton (+ All protons are exactly the same,all neutron (0) neutrons are exactly the same,and all electrons are exactly the same. There are many elements in the PERIODIC TABLE.Over 100! The thing that makes those elements different is the number of electrons,protons,and neutrons
All the matter around you is made of atoms, and all atoms are made of only three types of subatomic particle, protons, electrons, and neutrons. All protons are exactly the same, all neutrons are exactly the same, and all electrons are exactly the same. Atomic Structure There are many elements in the PERIODIC TABLE. Over 100! The thing that makes those elements different is the number of electrons, protons, and neutrons
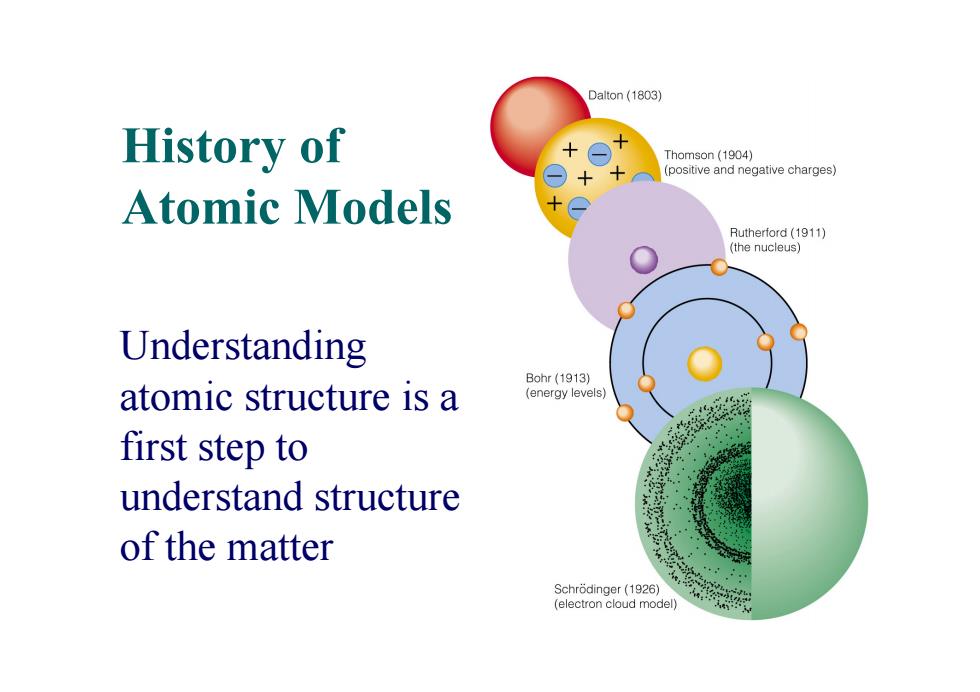
Dalton (1803) History of Thomson(1904) positive and negative charges) Atomic models Rutherford(1911) (the nucleus) Understanding Bohr(1913) atomic structure is a (energy levels) first step to understand structure of the matter Schrodinger(1926) (electron cloud model)
History of Atomic Models Understanding atomic structure is a first step to understand structure of the matter
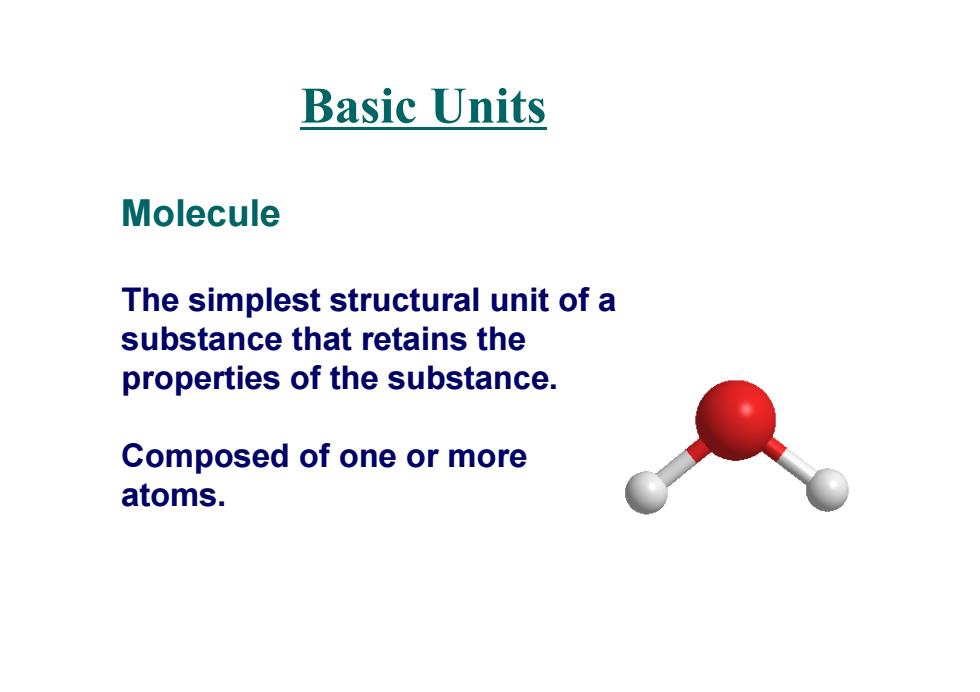
Basic Units Molecule The simplest structural unit of a substance that retains the properties of the substance. Composed of one or more atoms
Molecule The simplest structural unit of a substance that retains the properties of the substance. Composed of one or more atoms. Basic Units