正在加载图片...
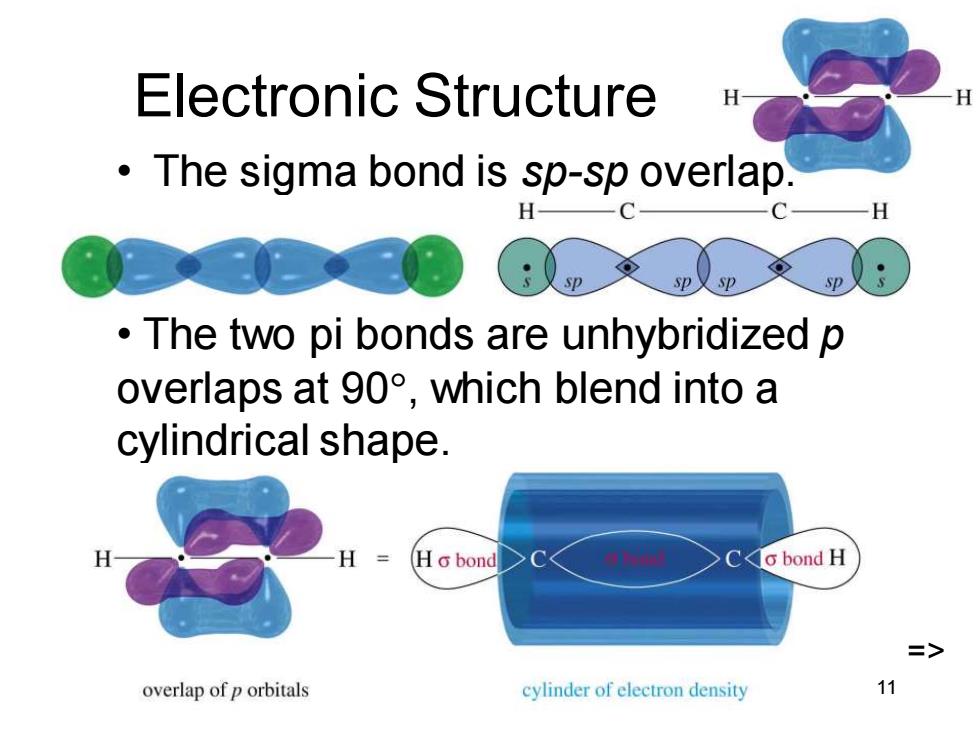
Electronic Structure The sigma bond is sp-sp overlap. C 一H p The two pi bonds are unhybridized p overlaps at90°,which blend into a cylindrical shape. HG bond Co bond H => overlap of p orbitals cylinder of electron density 11 Chapter 9 11 Electronic Structure • The sigma bond is sp-sp overlap. • The two pi bonds are unhybridized p overlaps at 90, which blend into a cylindrical shape. =>