正在加载图片...
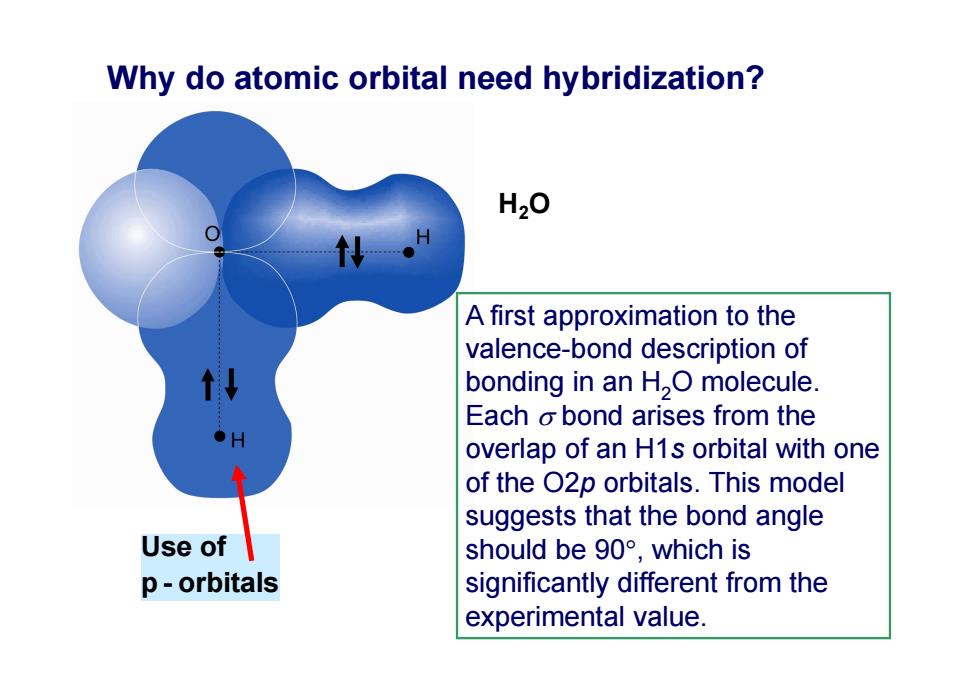
Why do atomic orbital need hybridization? H20 A first approximation to the valence-bond description of bonding in an H,O molecule. Each o bond arises from the overlap of an H1s orbital with one of the O2p orbitals.This model suggests that the bond angle Use of should be90°,which is p-orbitals significantly different from the experimental value.Use of p - orbitals H2O Why do atomic orbital need hybridization? A first approximation to the valence-bond description of bonding in an H2O molecule. Each bond arises from the overlap of an H1s orbital with one of the O2p orbitals. This model suggests that the bond angle should be 90, which is significantly different from the experimental value