正在加载图片...
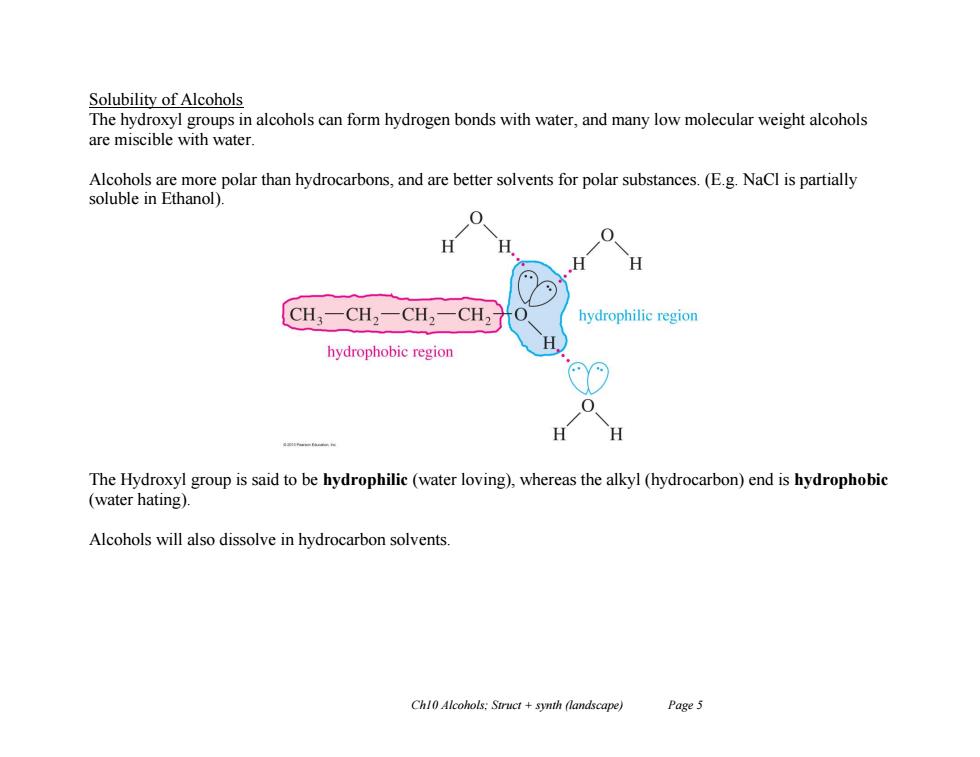
Solubility of Alcohols The hydroxyl groups in alcohols can form hydrogen bonds with water,and many low molecular weight alcohols are miscible with water Alcohols are more polar than hydrocarbons,and are better solvents for polar substances.(E.g.NaCl is partially soluble in Ethanol) H CH3一CH2一CH2一CH2 hydrophilic region hydrophobic region H H The Hydroxyl group is said to be hydrophilic(water loving),whereas the alkyl(hydrocarbon)end is hydrophobic (water hating). Alcohols will also dissolve in hydrocarbon solvents. Chl0 Alcohols:Struct +synth (landscape) Page 5 Ch10 Alcohols; Struct + synth (landscape) Page 5 Solubility of Alcohols The hydroxyl groups in alcohols can form hydrogen bonds with water, and many low molecular weight alcohols are miscible with water. Alcohols are more polar than hydrocarbons, and are better solvents for polar substances. (E.g. NaCl is partially soluble in Ethanol). The Hydroxyl group is said to be hydrophilic (water loving), whereas the alkyl (hydrocarbon) end is hydrophobic (water hating). Alcohols will also dissolve in hydrocarbon solvents