正在加载图片...
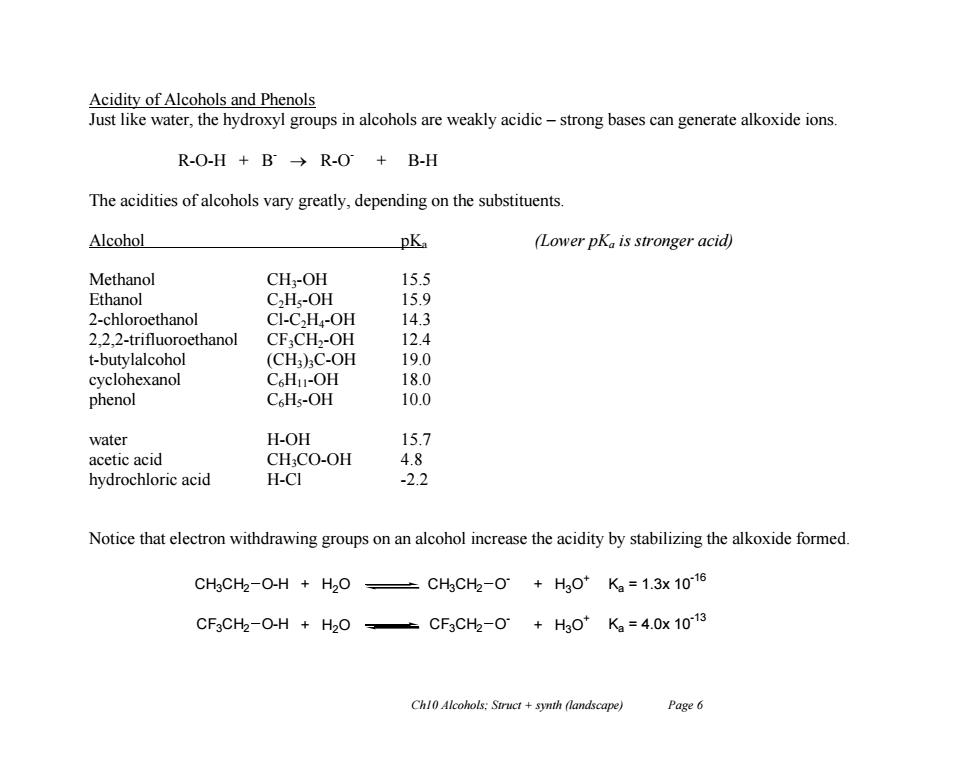
Acidity of Alcohols and Phenols Just like water,the hydroxyl groups in alcohols are weakly acidic-strong bases can generate alkoxide ions. R-O-H+B→R-O+B-H The acidities of alcohols vary greatly,depending on the substituents Alcohol pKa (Lower pKa is stronger acid) Methanol CH,-OH 15.5 Ethanol C,H;-OH 15.9 2-chloroethanol CI-C2H-OH 14.3 2,2,2-trifluoroethanol CFCH2-OH 12.4 t-butylalcohol (CH)C-OH 19.0 cyclohexanol C HI-OH 18.0 phenol C Hs-OH 10.0 water H-OH 15.7 acetic acid CHCO-OH 4.8 hydrochloric acid H-CI -2.2 Notice that electron withdrawing groups on an alcohol increase the acidity by stabilizing the alkoxide formed. CH3CH2-O-H H2O ±CHgCH2-0+H30*K2=1.3x1016 CF3CH2-O-H H2O -CF3CHh-O+H0*K2=4.0x1013 Chl0 Alcohols:Struct +synth (landscape) Page 6Ch10 Alcohols; Struct + synth (landscape) Page 6 Acidity of Alcohols and Phenols Just like water, the hydroxyl groups in alcohols are weakly acidic – strong bases can generate alkoxide ions. R-O-H + B- R-O - + B-H The acidities of alcohols vary greatly, depending on the substituents. Alcohol pKa (Lower pKa is stronger acid) Methanol CH3-OH 15.5 Ethanol C2H5-OH 15.9 2-chloroethanol Cl-C2H4-OH 14.3 2,2,2-trifluoroethanol CF3CH2-OH 12.4 t-butylalcohol (CH3)3C-OH 19.0 cyclohexanol C6H11-OH 18.0 phenol C6H5-OH 10.0 water H-OH 15.7 acetic acid CH3CO-OH 4.8 hydrochloric acid H-Cl -2.2 Notice that electron withdrawing groups on an alcohol increase the acidity by stabilizing the alkoxide formed. CH3CH2 O-H + H2O CH3CH2 O - + H3O + Ka = 1.3x 10 -16 CF3CH2 O-H + H2O CF3CH2 O - + H3O + Ka = 4.0x 10 -13