正在加载图片...
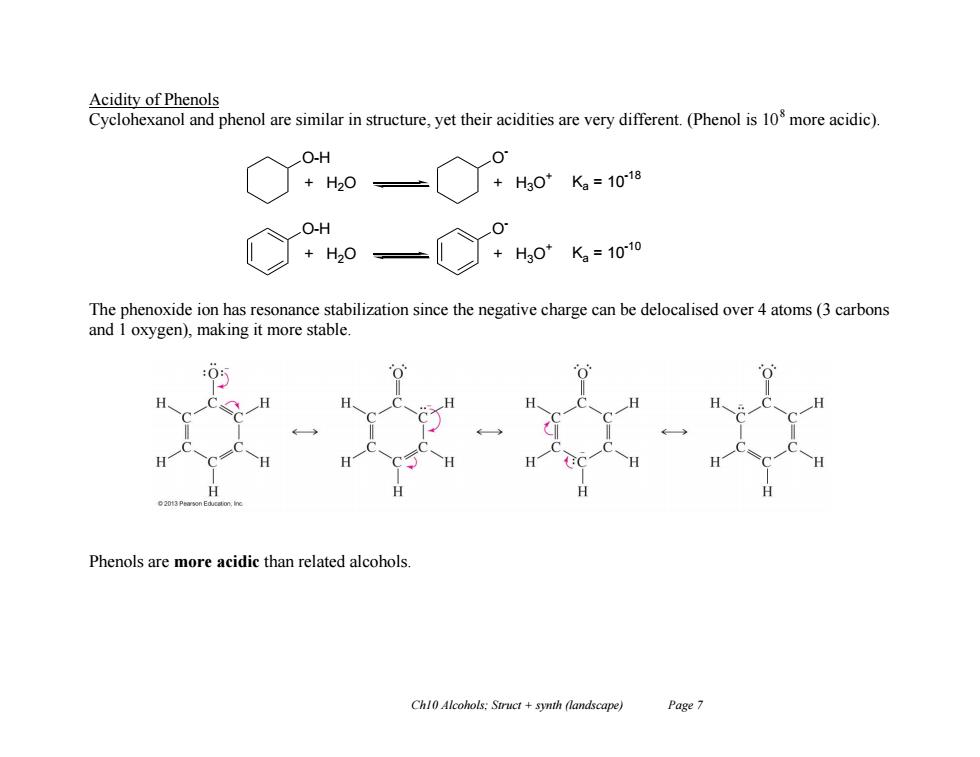
Acidity of Phenols Cyclohexanol and phenol are similar in structure,yet their acidities are very different.(Phenol is 10 more acidic). O-H +H20= +H3O* K2=1018 O-H +H20 +H30K2=1010 The phenoxide ion has resonance stabilization since the negative charge can be delocalised over 4 atoms(3 carbons and I oxygen),making it more stable. Phenols are more acidic than related alcohols. Chl0 Alcohols:Swruct +synth (landscape) Page 7 Ch10 Alcohols; Struct + synth (landscape) Page 7 Acidity of Phenols Cyclohexanol and phenol are similar in structure, yet their acidities are very different. (Phenol is 108 more acidic). The phenoxide ion has resonance stabilization since the negative charge can be delocalised over 4 atoms (3 carbons and 1 oxygen), making it more stable. Phenols are more acidic than related alcohols. O-H + H2O O - + H3O + Ka = 10 -18 O-H + H2O O - + H3O + Ka = 10 -10