正在加载图片...
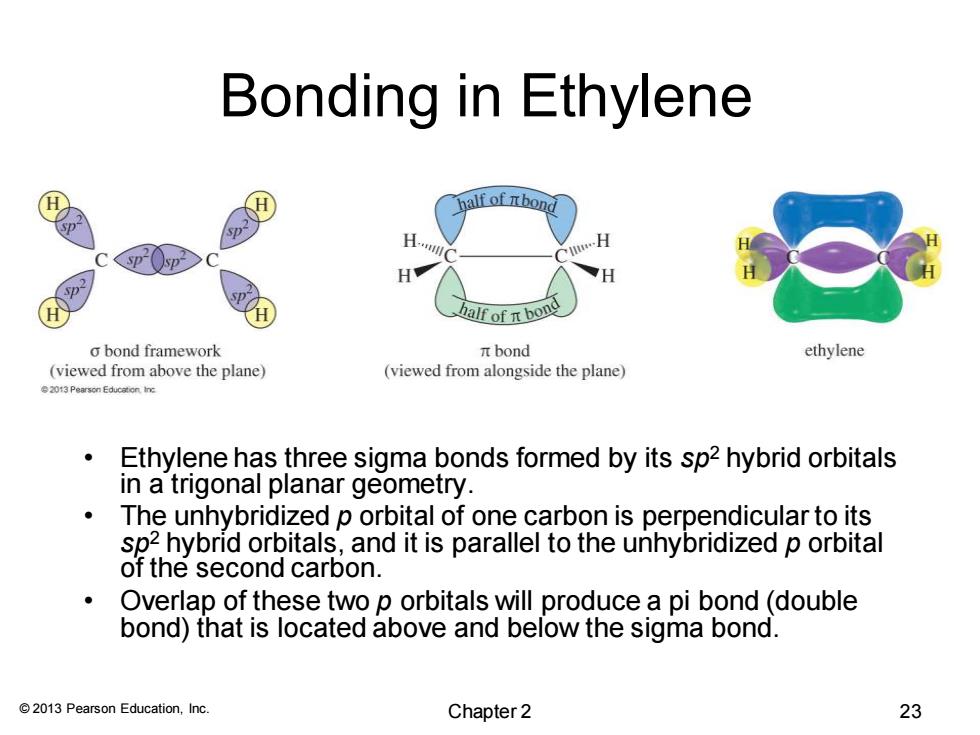
Bonding in Ethylene nalf of Ibond H H H half of n bone o bond framework πbond ethylene (viewed from above the plane) (viewed from alongside the plane) 2013 Pearson Education ine Ethylene has three sigma bonds formed by its sp2 hybrid orbitals in a trigonal planar geometry. The unhybridized p orbital of one carbon is perpendicular to its sp2 hybrid orbitals,and it is parallel to the unhybridized p orbital of the second carbon. Overlap of these two p orbitals will produce a pi bond(double bond)that is located above and below the sigma bond. 2013 Pearson Education,Inc. Chapter 2 23© 2013 Pearson Education, Inc. Bonding in Ethylene • Ethylene has three sigma bonds formed by its sp2 hybrid orbitals in a trigonal planar geometry. • The unhybridized p orbital of one carbon is perpendicular to its sp2 hybrid orbitals, and it is parallel to the unhybridized p orbital of the second carbon. • Overlap of these two p orbitals will produce a pi bond (double bond) that is located above and below the sigma bond. Chapter 2 23