正在加载图片...
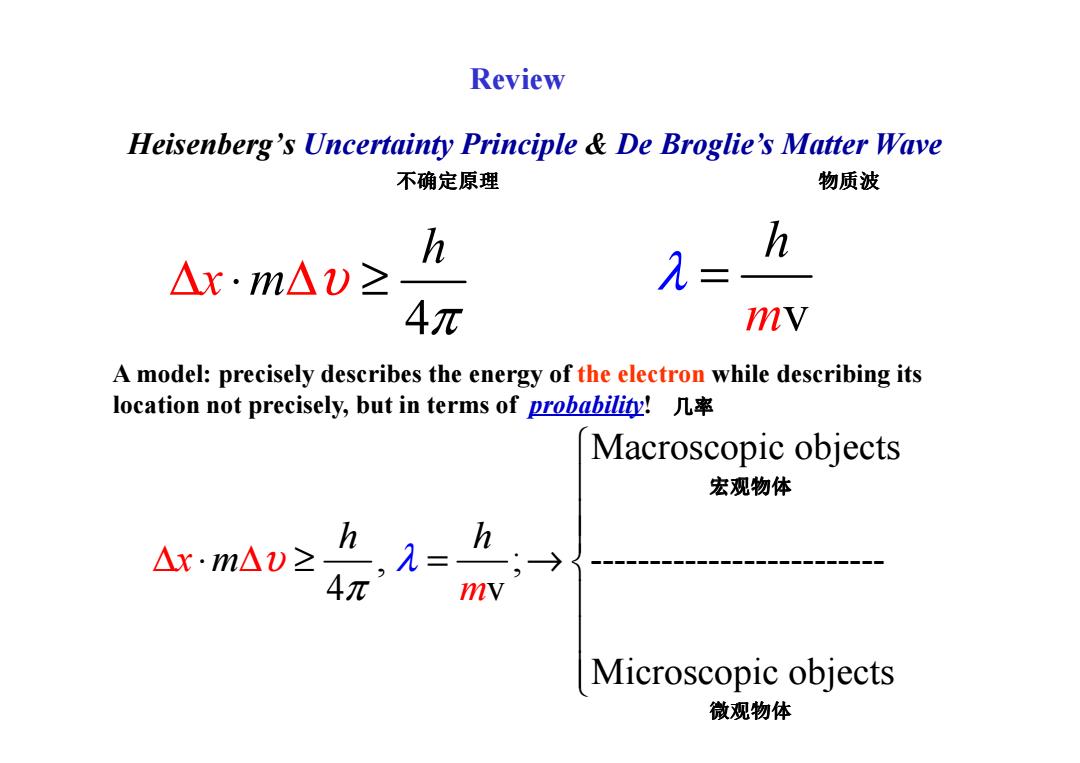
Review Heisenberg's Uncertainty Principle De Broglie's Matter Wave 不确定原理 物质波 h h △x·m△)≥ = 4元 mV A model:precisely describes the energy of the electron while describing its location not precisely,but in terms of probability! Macroscopic objects 宏观物体 h △x·m△v≥ h,= mv Microscopic objects 微观物体Heisenberg’s Uncertainty Principle & De Broglie’s Matter Wave 4 h x m υ π ∆ ⋅ ∆ ≥ mv h λ = A model: precisely describes the energy of the electron while describing its 不确定原理 物质波 Review Macroscopic objects , ; ------------------------- 4 v Microscopic objects x h m h m υ π λ ∆ ∆ ⋅ ≥ = → A model: precisely describes the energy of the electron while describing its location not precisely, but in terms of probability! 几率 宏观物体 微观物体