正在加载图片...
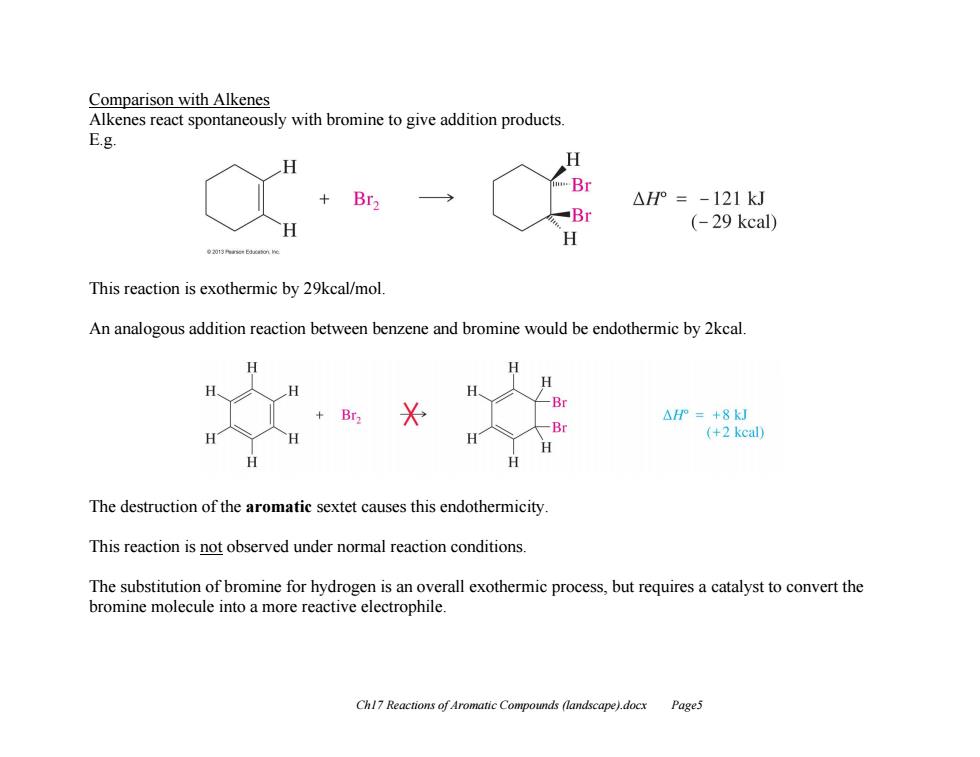
Comparison with Alkenes Alkenes react spontaneously with bromine to give addition products. E.g. H Br2 Br △H°=-121kJ Br (-29 kcal) This reaction is exothermic by 29kcal/mol. An analogous addition reaction between benzene and bromine would be endothermic by 2kcal. H Br △HP=+8kJ -Br (+2kc) The destruction of the aromatic sextet causes this endothermicity This reaction is not observed under normal reaction conditions. The substitution of bromine for hydrogen is an overall exothermic process,but requires a catalyst to convert the bromine molecule into a more reactive electrophile. Chl7 Reactions of Aromatic Compounds (landscape).docx Page5Ch17 Reactions of Aromatic Compounds (landscape).docx Page5 Comparison with Alkenes Alkenes react spontaneously with bromine to give addition products. E.g. This reaction is exothermic by 29kcal/mol. An analogous addition reaction between benzene and bromine would be endothermic by 2kcal. The destruction of the aromatic sextet causes this endothermicity. This reaction is not observed under normal reaction conditions. The substitution of bromine for hydrogen is an overall exothermic process, but requires a catalyst to convert the bromine molecule into a more reactive electrophile